Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions
- PMID: 19755485
- PMCID: PMC2791494
- DOI: 10.1530/REP-09-0300
Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions
Abstract
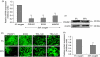
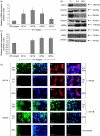
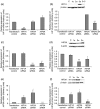
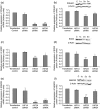
Human embryonic stem (hES) cells are routinely cultured under atmospheric, 20% oxygen tensions but are derived from embryos which reside in a 3-5% oxygen (hypoxic) environment. Maintenance of oxygen homeostasis is critical to ensure sufficient levels for oxygen-dependent processes. This study investigates the importance of specific hypoxia inducible factors (HIFs) in regulating the hypoxic responses of hES cells. We report that culture at 20% oxygen decreased hES cell proliferation and resulted in a significantly reduced expression of SOX2, NANOG and POU5F1 (OCT4) mRNA as well as POU5F1 protein compared with hypoxic conditions. HIF1A protein was not expressed at 20% oxygen and displayed only a transient, nuclear localisation at 5% oxygen. HIF2A (EPAS1) and HIF3A displayed a cytoplasmic localisation during initial hypoxic culture but translocated to the nucleus following long-term culture at 5% oxygen and were significantly upregulated compared with cells cultured at 20% oxygen. Silencing of HIF2A resulted in a significant decrease in both hES cell proliferation and POU5F1, SOX2 and NANOG protein expression while the early differentiation marker, SSEA1, was concomitantly increased. HIF3A upregulated HIF2A and prevented HIF1A expression with the knockdown of HIF3A resulting in the reappearance of HIF1A protein. In summary, these data demonstrate that a low oxygen tension is preferential for the maintenance of a highly proliferative, pluripotent population of hES cells. While HIF3A was found to regulate the expression of both HIF1A and HIF2A, it is HIF2A which regulates hES cell pluripotency as well as proliferation under hypoxic conditions.
Figures

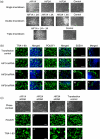
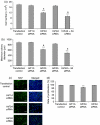
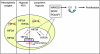
References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Cameron CM, Harding F, Hu WS, Kaufman DS. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Experimental Biology and Medicine. 2008;233:1044–1057. - PubMed
-
- Chen HF, Kuo HC, Chen W, Wu FC, Yang YS, Ho HN. A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Human Reproduction. 2009;1:71–80. - PubMed
-
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature Medicine. 2002;8:702–710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0701153/MRC_/Medical Research Council/United Kingdom
- BB/G006970/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT066492MA/WT_/Wellcome Trust/United Kingdom
- 088566/WT_/Wellcome Trust/United Kingdom
- BB/G010579/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

