Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin
- PMID: 19755493
- PMCID: PMC2758802
- DOI: 10.1242/jcs.045674
Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin
Abstract
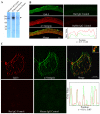
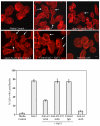
Recent studies have shown that galectin-3 (Gal-3; also known as LGALS3), a beta-galactoside-binding lectin, promotes cell migration during re-epithelialization of corneal wounds. The goal of this study was to characterize the molecular mechanism by which Gal-3 stimulates cell migration. We demonstrate here that exogenous Gal-3, but not Gal-1 or Gal-8, promotes cell scattering and formation of lamellipodia in human corneal epithelial cells in a beta-lactose-inhibitable manner. alpha3beta1 integrin was identified as the major Gal-3-binding protein in corneal epithelial cells by affinity chromatography of cell lysates on a Gal-3-Sepharose column. Preincubation of cells with anti-alpha3 integrin function-blocking antibody significantly inhibited the induction of lamellipodia by Gal-3. Furthermore, exogenous Gal-3 activated both focal adhesion kinase, a key regulator of integrin-dependent intracellular signaling, and Rac1 GTPase, a member of the family of Rho GTPases, well known for its role in the reorganization of the actin cytoskeleton and formation of lamellipodial extensions. Experiments involving knockdown of beta-1,6-N-acetylglucosaminytransferase V, an enzyme that synthesizes high-affinity glycan ligands for Gal-3, revealed that carbohydrate-mediated interaction between Gal-3 and complex N-glycans on alpha3beta1 integrin plays a key role in Gal-3-induced lamellipodia formation. We propose that Gal-3 promotes epithelial cell migration by cross-linking MGAT5-modified complex N-glycans on alpha3beta1 integrin and subsequently activating alpha3beta1-integrin-Rac1 signaling to promote lamellipodia formation.
Figures





References
-
- Bao, Q. and Hughes, R. C. (1999). Galectin-3 and polarized growth within collagen gels of wild-type and ricin-resistant MDCK renal epithelial cells. Glycobiology 9, 489-495. - PubMed
-
- Bellis, S. L. (2004). Variant glycosylation: an underappreciated regulatory mechanism for beta1 integrins. Biochim. Biophys. Acta 1663, 52-60. - PubMed
-
- Brewer, C. F. (2004). Thermodynamic binding studies of galectin-1, Gal-3 and -7. Glycoconj. J. 19, 459-465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

