Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity
- PMID: 19755710
- PMCID: PMC2850287
- DOI: 10.1126/scisignal.2000370
Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity
Abstract
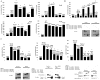
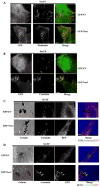
The mechanisms that determine localized formation of reactive oxygen species (ROS) through NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase (Nox) family members in nonphagocytic cells are unknown. We show that the c-Src substrate proteins Tks4 (tyrosine kinase substrate with four SH3 domains) and Tks5 are functional members of a p47(phox)-related organizer superfamily. Tks proteins selectively support Nox1 and Nox3 (and not Nox2 and Nox4) activity in reconstituted cellular systems and interact with the NoxA1 activator protein through an Src homology 3 domain-mediated interaction. Endogenous Tks4 is required for Rac guanosine triphosphatase- and Nox1-dependent ROS production by DLD1 colon cancer cells. Our results are consistent with the Tks-mediated recruitment of Nox1 to invadopodia that form in DLD1 cells in a Tks- and Nox-dependent fashion. We propose that Tks organizers represent previously unrecognized members of an organizer superfamily that link Nox to localized ROS formation.
Figures




References
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245. - PubMed
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181. - PubMed
-
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005. - PubMed
-
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

