Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis
- PMID: 19755711
- PMCID: PMC2884999
- DOI: 10.1126/scisignal.2000304
Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis
Abstract
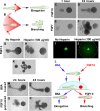
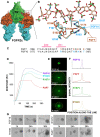
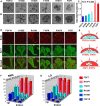
The developmental activities of morphogens depend on the gradients that they form in the extracellular matrix. Here, we show that differences in the binding of fibroblast growth factor 7 (FGF7) and FGF10 to heparan sulfate (HS) underlie the formation of different gradients that dictate distinct activities during branching morphogenesis. Reducing the binding affinity of FGF10 for HS by mutating a single residue in its HS-binding pocket converted FGF10 into a functional mimic of FGF7 with respect to gradient formation and regulation of branching morphogenesis. In particular, the mutant form of FGF10 caused lacrimal and salivary gland epithelium buds to branch rather than to elongate. In contrast, mutations that reduced the affinity of the FGF10 for its receptor affected the extent, but not the nature, of the response. Our data may provide a general model for understanding how binding to HS regulates other morphogenetic gradients.
Figures

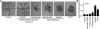
References
-
- Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005;15:506–516. - PubMed
-
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. - PubMed
-
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. - PubMed
-
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–750. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

