Copper-triggered aggregation of ubiquitin
- PMID: 19756145
- PMCID: PMC2737635
- DOI: 10.1371/journal.pone.0007052
Copper-triggered aggregation of ubiquitin
Abstract
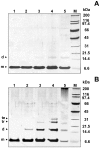
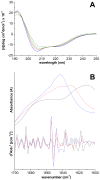
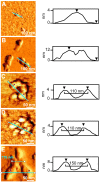
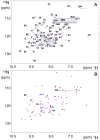
Neurodegenerative disorders share common features comprising aggregation of misfolded proteins, failure of the ubiquitin-proteasome system, and increased levels of metal ions in the brain. Protein aggregates within affected cells often contain ubiquitin, however no report has focused on the aggregation propensity of this protein. Recently it was shown that copper, differently from zinc, nickel, aluminum, or cadmium, compromises ubiquitin stability and binds to the N-terminus with 0.1 micromolar affinity. This paper addresses the role of copper upon ubiquitin aggregation. In water, incubation with Cu(II) leads to formation of spherical particles that can progress from dimers to larger conglomerates. These spherical oligomers are SDS-resistant and are destroyed upon Cu(II) chelation or reduction to Cu(I). In water/trifluoroethanol (80:20, v/v), a mimic of the local decrease in dielectric constant experienced in proximity to a membrane surface, ubiquitin incubation with Cu(II) causes time-dependent changes in circular dichroism and Fourier-transform infrared spectra, indicative of increasing beta-sheet content. Analysis by atomic force and transmission electron microscopy reveals, in the given order, formation of spherical particles consistent with the size of early oligomers detected by gel electrophoresis, clustering of these particles in straight and curved chains, formation of ring structures, growth of trigonal branches from the rings, coalescence of the trigonal branched structures in a network. Notably, none of these ubiquitin aggregates was positive to tests for amyloid and Cu(II) chelation or reduction produced aggregate disassembly. The early formed Cu(II)-stabilized spherical oligomers, when reconstituted in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) liposomes and in POPC planar bilayers, form annular and pore-like structures, respectively, which are common to several neurodegenerative disorders including Parkinson's, Alzheimer's, amyotrophic lateral sclerosis, and prion diseases, and have been proposed to be the primary toxic species. Susceptibility to aggregation of ubiquitin, as it emerges from the present study, may represent a potential risk factor for disease onset or progression while cells attempt to tag and process toxic substrates.
Conflict of interest statement
Figures




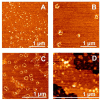
References
-
- McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7:736–741. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. - PubMed
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

