Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis
- PMID: 19756557
- PMCID: PMC2778780
- DOI: 10.1007/s00228-009-0718-4
Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis
Abstract
Purpose: Infliximab, a monoclonal antibody, is approved for the treatment of inflammatory diseases at doses that depend on the patient disease population. It was the aim of this study to evaluate its population pharmacokinetics in patients with moderately to severely active ulcerative colitis and characterize patient covariates that affect its disposition in this population.
Methods: Information collected from 482 patients in two randomized, double-blind, placebo-controlled international studies were analyzed using NONMEM.
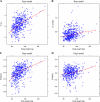
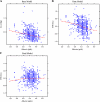
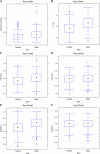
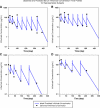
Results: A two-compartment, population pharmacokinetic model described the serum infliximab concentration-time data. Population pharmacokinetic estimates (typical value +/- standard error), based on the final covariate model, were clearance (CL: 0.407 +/- 0.0103 L/day), apparent volumes of distribution in the central (V(1): 3.29 +/- 0.0679 L) and peripheral (V(2): 4.13 +/- 0.16 L) compartments, and intercompartment clearance (Q: 7.14 +/- 0.489 L/day). Infliximab exhibited interindividual variability for CL and V(1) of 37.7% and 22.1%, respectively. Infliximab t(1/2) is approximately 14 days. Covariate analysis showed that V(1) increased as body weight increased, and CL was higher in patients who developed antibodies to infliximab. An additional novel covariate, serum albumin concentration, was found to be inversely and strongly related to infliximab clearance in this population.
Conclusions: The disposition of infliximab in patients with moderately to severely active ulcerative colitis, unlike in rheumatoid arthritis, was not affected by coadministration of immunomodulators and corticosteroids but was related to formation of antibodies to infliximab and, notably, to serum albumin levels.
Figures



References
-
- Remicade (infliximab) for IV injection. Malvern, Pa: Centocor Ortho Biotech, Inc., April 2009 (package insert)
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
-
- Gottlieb AB, Masud S, Ramamurthi R, Abdulghani A, Romano P, Chaudhari U, Dooley LT, Fasanmade A, Wagner CL. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-α monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. J Am Acad Dermatol. 2003;48:68–75. doi: 10.1067/mjd.2003.10. - DOI - PubMed
-
- Ternant D, Aubourg A, Magdelaine-Beuzelin C, Degenne D, Watier H, Picon L, Paintaud G. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit. 2008;30:523–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

