Apoptosis- and necrosis-induced changes in light attenuation measured by optical coherence tomography
- PMID: 19756838
- PMCID: PMC2807596
- DOI: 10.1007/s10103-009-0723-y
Apoptosis- and necrosis-induced changes in light attenuation measured by optical coherence tomography
Abstract
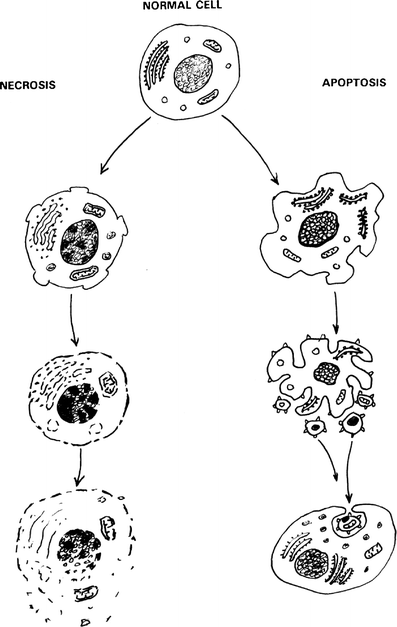
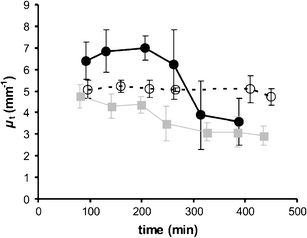
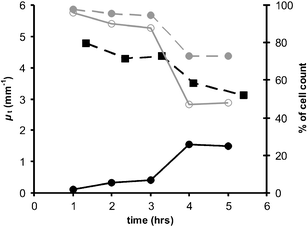
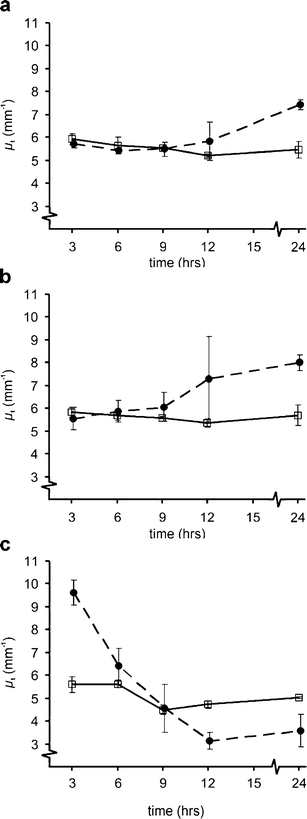
Optical coherence tomography (OCT) was used to determine optical properties of pelleted human fibroblasts in which necrosis or apoptosis had been induced. We analysed the OCT data, including both the scattering properties of the medium and the axial point spread function of the OCT system. The optical attenuation coefficient in necrotic cells decreased from 2.2 +/- 0.3 mm(1) to 1.3 +/- 0.6 mm(-1), whereas, in the apoptotic cells, an increase to 6.4 +/- 1.7 mm(-1) was observed. The results from cultured cells, as presented in this study, indicate the ability of OCT to detect and differentiate between viable, apoptotic, and necrotic cells, based on their attenuation coefficient. This functional supplement to high-resolution OCT imaging can be of great clinical benefit, enabling on-line monitoring of tissues, e.g. for feedback in cancer treatment.
Figures


References
-
- Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Darkes M, Robbins RC, Maecker HT, Strauss HW. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci U S A. 1998;95:6349–6354. doi: 10.1073/pnas.95.11.6349. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

