At the biological modeling and simulation frontier
- PMID: 19756975
- PMCID: PMC2763179
- DOI: 10.1007/s11095-009-9958-3
At the biological modeling and simulation frontier
Abstract
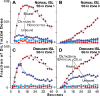
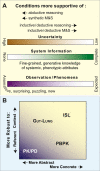
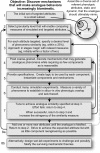
We provide a rationale for and describe examples of synthetic modeling and simulation (M&S) of biological systems. We explain how synthetic methods are distinct from familiar inductive methods. Synthetic M&S is a means to better understand the mechanisms that generate normal and disease-related phenomena observed in research, and how compounds of interest interact with them to alter phenomena. An objective is to build better, working hypotheses of plausible mechanisms. A synthetic model is an extant hypothesis: execution produces an observable mechanism and phenomena. Mobile objects representing compounds carry information enabling components to distinguish between them and react accordingly when different compounds are studied simultaneously. We argue that the familiar inductive approaches contribute to the general inefficiencies being experienced by pharmaceutical R&D, and that use of synthetic approaches accelerates and improves R&D decision-making and thus the drug development process. A reason is that synthetic models encourage and facilitate abductive scientific reasoning, a primary means of knowledge creation and creative cognition. When synthetic models are executed, we observe different aspects of knowledge in action from different perspectives. These models can be tuned to reflect differences in experimental conditions and individuals, making translational research more concrete while moving us closer to personalized medicine.
Figures