PDEs1-5 activity and expression in tissues of cirrhotic rats reveal a role for aortic PDE3 in NO desensitization
- PMID: 19758418
- PMCID: PMC2803251
- DOI: 10.1111/j.1365-2613.2009.00678.x
PDEs1-5 activity and expression in tissues of cirrhotic rats reveal a role for aortic PDE3 in NO desensitization
Abstract
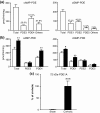
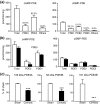
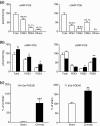
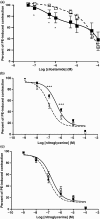
Liver cirrhosis is associated with increased nitric oxide (NO) production in the vasculature. We have previously demonstrated that aorta from rats with liver cirrhosis have a reduced relaxant response to NO donors that is corrected by DMPPO, a PDE5-specific inhibitor. Vasodilator responses to DMPPO itself were also reduced in rings from cirrhotic rats. These results supported previous suggestions that upregulation of PDE5 in liver cirrhosis might contribute to renal sodium retention, and consequently modulate vascular reactivity in the context of increased NO production (Tahseldar-Roumieh et al. in Am. J. Physiol. Heart Circ. Physiol. 290, H481-H488, 2006). Here, we investigated the possible alteration in activity and expression of cyclic nucleotide phosphodiesterase PDE1-PDE5 in kidney and vascular tissues in rats 4 weeks after bile duct ligation. The kidney of rats with cirrhosis had increased activity of PDE1 and PDE4 but not PDE5, and increased expression of PDE1A. Unexpectedly and interestingly, there was no change in cirrhotic aorta PDE5, but an increase in PDE3 and PDE4 activity associated with increased expression of PDE3A and PDE3B. Cilostamide, a specific PDE3 inhibitor, corrected the decreased response to an NO donor in isolated aorta from cirrhotic rats, suggesting that the difference in response to NO donors was due to differences in PDE3-induced hydrolysis of cGMP or to cGMP-induced inhibition of PDE3, rather than to differences in PDE5 contribution. In conclusion, these changes in PDE isozymes could greatly contribute to NO desensitization and to the regulation of vascular and renal function in liver cirrhosis.
Figures

References
-
- Ahloulay M, Bankir L, Lugnier C, et al. Cyclic AMP-phosphodiesterases inhibitor improves sodium excretion in rats with cirrhosis and ascites. Liver Int. 2005;25:403–409. - PubMed
-
- Angeli P, Jimenez W, Veggian R, et al. Increased activity of guanosine 3′-5′-cyclic monophosphate phosphodiesterase in the renal tissue of cirrhotic rats with ascites. Hepatology. 2000;31:304–310. - PubMed
-
- Atucha NM, Nadal FJ, Iyu D, et al. Role of vascular nitric oxide in experimental liver cirrhosis. Curr. Vasc. Pharmacol. 2005;3:81–85. - PubMed
-
- Bernardi M, Di Marco C, Trevisani F, et al. Renal sodium retention during upright posture in preascitic cirrhosis. Gastroenterology. 1993;105:188–193. - PubMed
-
- Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol. Ther. 2001;89:221–231. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

