Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK
- PMID: 19758428
- PMCID: PMC2760530
- DOI: 10.1186/1471-2458-9-342
Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK
Abstract
Background: Diabetes prevalence is increasing. The Finnish Diabetes Prevention Study (DPS) showed a 58% reduction in Type 2 Diabetes (T2D) incidence in adults with impaired glucose tolerance (IGT). The European Diabetes Prevention Study (EDIPS) extends the DPS to different European populations, using the same study design. In the Newcastle arm of this study (EDIPS-Newcastle), we tested the hypothesis that T2D can be prevented by lifestyle intervention and explored secondary outcomes in relation to diabetes incidence.
Methods: We recruited 102 participants (42 men and 60 women, mean age 57 years, mean BMI 34 kgm-2) with IGT to EDIPS-Newcastle and randomised to Intervention and usual care Control groups. The intervention included individual motivational interviewing aimed at: weight reduction, increase in physical activity, fibre and carbohydrate intake and reduction of fat intake (secondary outcomes). The primary outcome was diagnosis of T2D.
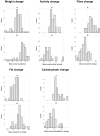
Results: Mean duration of follow-up was 3.1 years. T2D was diagnosed in 16 participants (I = 5, C = 11). Absolute incidence of T2D was 32.7 per 1000 person-years in the Intervention-group and 67.1 per 1000 person-years in the Control-group. The overall incidence of diabetes was reduced by 55% in the Intervention-group, compared with the Control-group: RR 0.45 (95%CI 0.2 to 1.2).Explanatory survival analysis of secondary outcomes showed that those who sustained beneficial changes for two or more years reduced their risk of developing T2D.
Conclusion: Our results are consistent with other diabetes prevention trials. This study was designed as part of a larger study and although the sample size limits statistical significance, the results contribute to the evidence that T2D can be prevented by lifestyle changes in adults with IGT. In explanatory analysis small sustained beneficial changes in weight, physical activity or dietary factors were associated with reduction in T2D incidence.
Trial registration: International Standard Randomised Controlled Trial Number registry (ISRCTN) Registry number: ISRCTN15670600. (http://www.controlled-trials.com/isrctn/search.html?srch=15670600&sort=3&dir=desc&max=10).
Figures



References
-
- International Diabetes Federation . Diabetes Atlas. 3. Brussels: International Diabetes Federation; 2006.
-
- Roberts S. Improving Diabetes Services, the National Service Framework for Diabetes two years on. Edited by Health Do. London: TSO; 2005.
-
- Saad MF, Knowler WC, Pettit DJ, Nelson RG, MA C, et al. A two step model for the development of non-insulin dependent diabetes mellitus. American Journal of Medicine. 1991;90:229–235. - PubMed
-
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

