Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'
- PMID: 19758979
- PMCID: PMC2816191
- DOI: 10.1093/humupd/dmp032
Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'
Abstract
Background: Functional male gametes are produced through complex processes that take place within the testis, epididymis and female reproductive tract. A breakdown at any of these phases can result in male infertility. The production of mutant mouse models often yields an unexpected male infertility phenotype. It is with this in mind that the current review has been written. The review aims to act as a guide to the 'non-reproductive biologist' to facilitate a systematic analysis of sterile or subfertile mice and to assist in extracting the maximum amount of information from each model.
Methods: This is a review of the original literature on defects in the processes that take a mouse spermatogonial stem cell through to a fully functional spermatozoon, which result in male infertility. Based on literature searches and personal experience, we have outlined a step-by-step strategy for the analysis of an infertile male mouse line.
Results: A wide range of methods can be used to define the phenotype of an infertile male mouse. These methods range from histological methods such as electron microscopy and immunohistochemistry, to hormone analyses and methods to assess sperm maturation status and functional competence.
Conclusion: With the increased rate of genetically modified mouse production, the generation of mouse models with unexpected male infertility is increasing. This manuscript will help to ensure that the maximum amount of information is obtained from each mouse model and, by extension, will facilitate the knowledge of both normal fertility processes and the causes of human infertility.
Figures
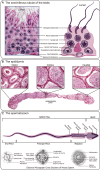
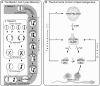
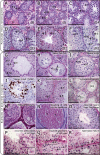
References
-
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. - PubMed
-
- Abou-Haila A, Fain-Maurel MA. Regional differences of the proximal part of mouse epididymis: morphological and histochemical characterization. Anat Rec. 1984;209:197–208. - PubMed
-
- Abou-Haila A, Tulsiani DR. Mammalian sperm acrosome: formation, contents, and function. Arch Biochem Biophys. 2000;379:173–182. - PubMed
-
- Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108:2017–2025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

