Bax1 is a novel endonuclease: implications for archaeal nucleotide excision repair
- PMID: 19759013
- PMCID: PMC2781640
- DOI: 10.1074/jbc.M109.055913
Bax1 is a novel endonuclease: implications for archaeal nucleotide excision repair
Abstract
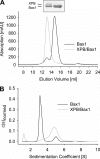
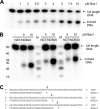
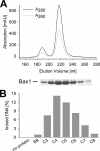
The helicases XPB and XPD are part of the TFIIH complex, which mediates transcription initiation as well as eukaryotic nucleotide excision repair (NER). Although there is no TFIIH complex present in archaea, most species contain both XPB and XPD and serve as a model for their eukaryotic homologs. Recently, a novel binding partner for XPB, Bax1 (binds archeal XPB), was identified in archaea. To gain insights into its role in NER, Bax1 from Thermoplasma acidophilum was characterized. We identified Bax1 as a novel Mg(2+)-dependent structure-specific endonuclease recognizing DNA containing a 3' overhang. Incision assays conducted with DNA substrates providing different lengths of the 3' overhang indicate that Bax1 specifically incises DNA in the single-stranded region of the 3' overhang 4-6 nucleotides to the single-stranded DNA/double-stranded DNA junction and thus is a structure-specific and not a sequence-specific endonuclease. In contrast, no incision was detected in the presence of a 5' overhang, double-stranded DNA, or DNA containing few unpaired nucleotides forming a bubble. Several Bax1 variants were generated based on multiple sequence alignments and examined with respect to their ability to perform the incision reaction. Residues Glu-124, Asp-132, Tyr-152, and Glu-155 show a dramatic reduction in incision activity, indicating a pivotal role in catalysis. Interestingly, Bax1 does not exhibit any incision activity in the presence of XPB, thus suggesting a role in NER in which the endonuclease activity is tightly regulated until the damage has been recognized and verified prior to the incision event.
Figures
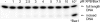

Similar articles
-
The XBP-Bax1 helicase-nuclease complex unwinds and cleaves DNA: implications for eukaryal and archaeal nucleotide excision repair.J Biol Chem. 2010 Apr 2;285(14):11013-22. doi: 10.1074/jbc.M109.094763. Epub 2010 Feb 6. J Biol Chem. 2010. PMID: 20139443 Free PMC article.
-
XPB helicase regulates DNA incision by the Thermoplasma acidophilum endonuclease Bax1.DNA Repair (Amst). 2012 Mar 1;11(3):286-93. doi: 10.1016/j.dnarep.2011.12.002. Epub 2012 Jan 9. DNA Repair (Amst). 2012. PMID: 22237014
-
The archaeal XPB protein is a ssDNA-dependent ATPase with a novel partner.J Mol Biol. 2008 Feb 22;376(3):634-44. doi: 10.1016/j.jmb.2007.12.019. Epub 2007 Dec 15. J Mol Biol. 2008. PMID: 18177890
-
Helicases required for nucleotide excision repair: structure, function and mechanism.Enzymes. 2023;54:273-304. doi: 10.1016/bs.enz.2023.05.002. Epub 2023 Jun 3. Enzymes. 2023. PMID: 37945175 Review.
-
Three-dimensional structural views of damaged-DNA recognition: T4 endonuclease V, E. coli Vsr protein, and human nucleotide excision repair factor XPA.Mutat Res. 2000 Aug 30;460(3-4):257-75. doi: 10.1016/s0921-8777(00)00031-8. Mutat Res. 2000. PMID: 10946233 Review.
Cited by
-
Structural basis of the XPB helicase-Bax1 nuclease complex interacting with the repair bubble DNA.Nucleic Acids Res. 2020 Nov 18;48(20):11695-11705. doi: 10.1093/nar/gkaa801. Nucleic Acids Res. 2020. PMID: 32986831 Free PMC article.
-
Single-stranded DNA binding activity of XPBI, but not XPBII, from Sulfolobus tokodaii causes double-stranded DNA melting.Extremophiles. 2011 Jan;15(1):67-76. doi: 10.1007/s00792-010-0338-z. Epub 2010 Dec 5. Extremophiles. 2011. PMID: 21132514
-
Transfer RNA methyltransferases from Thermoplasma acidophilum, a thermoacidophilic archaeon.Int J Mol Sci. 2014 Dec 23;16(1):91-113. doi: 10.3390/ijms16010091. Int J Mol Sci. 2014. PMID: 25546389 Free PMC article.
-
Structural basis of the XPB-Bax1 complex as a dynamic helicase-nuclease machinery for DNA repair.Nucleic Acids Res. 2020 Jun 19;48(11):6326-6339. doi: 10.1093/nar/gkaa324. Nucleic Acids Res. 2020. PMID: 32374860 Free PMC article.
-
The XBP-Bax1 helicase-nuclease complex unwinds and cleaves DNA: implications for eukaryal and archaeal nucleotide excision repair.J Biol Chem. 2010 Apr 2;285(14):11013-22. doi: 10.1074/jbc.M109.094763. Epub 2010 Feb 6. J Biol Chem. 2010. PMID: 20139443 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

