Reticuloendotheliosis virus strain T induces miR-155, which targets JARID2 and promotes cell survival
- PMID: 19759154
- PMCID: PMC2786729
- DOI: 10.1128/JVI.01182-09
Reticuloendotheliosis virus strain T induces miR-155, which targets JARID2 and promotes cell survival
Abstract
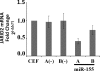
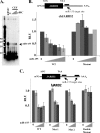
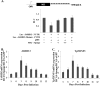
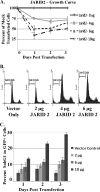
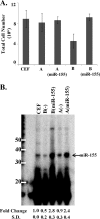
The oncogenic microRNA miR-155 is upregulated by several oncogenic viruses. The precursor of miR-155, termed bic, was first observed to cooperate with myc in chicken B-cell lymphomas induced by avian leukosis proviral integrations. We identified another oncogenic retrovirus, reticuloendotheliosis virus strain T (REV-T), that upregulates miR-155 in chicken embryo fibroblasts. We also observed very high levels of miR-155 in REV-T-induced B-cell lymphomas. To study the role of miR-155 in these tumors, we identified JARID2/Jumonji, a cell cycle regulator and part of a histone methyltransferase complex, as a target of miR-155. The overexpression of miR-155 decreased levels of endogenous JARID2 mRNA. We confirmed that miR-155 directly targets both human and chicken JARID2 by assaying the repression of reporters containing the JARID2 3'-untranslated regions. Further, the overexpression of a sponge complementary to miR-155 in a tumor cell line increased endogenous JARID2 mRNA levels. The overexpression of JARID2 in chicken fibroblasts led to decreased cell numbers and an increase in apoptotic cells. The overexpression of miR-155 rescued cells undergoing cytopathic effect caused by infection with subgroup B avian retroviruses. Therefore, we propose that miR-155 has a prosurvival function that is mediated through the downregulation of targets including JARID2.
Figures


References
-
- Bagga, S., J. Bracht, S. Hunter, K. Massirer, J. Holtz, R. Eachus, and A. E. Pasquinelli. 2005. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122:553-563. - PubMed
-
- Bouchard, C., P. Staller, and M. Eilers. 1998. Control of cell proliferation by Myc. Trends Cell Biol. 8:202-206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

