Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial
- PMID: 19759166
- PMCID: PMC2762163
- DOI: 10.3945/ajcn.2009.28001
Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial
Abstract
Background: Isoflavones are naturally occurring plant estrogens that are abundant in soy. Although purported to protect against bone loss, the efficacy of soy isoflavone supplementation in the prevention of osteoporosis in postmenopausal women remains controversial.
Objective: Our aim was to test the effect of soy isoflavone supplementation on bone health.
Design: A multicenter, randomized, double-blind, placebo-controlled 24-mo trial was conducted to assess the effects of daily supplementation with 80 or 120 mg of soy hypocotyl aglycone isoflavones plus calcium and vitamin D on bone changes in 403 postmenopausal women. Study subjects were tested annually and changes in whole-body and regional bone mineral density (BMD), bone mineral content (BMC), and T scores were assessed. Changes in serum biochemical markers of bone metabolism were also assessed.
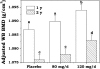
Results: After study site, soy intake, and pretreatment values were controlled for, subjects receiving a daily supplement with 120 mg soy isoflavones had a statistically significant smaller reduction in whole-body BMD than did the placebo group both at 1 y (P < 0.03) and at 2 y (P < 0.05) of treatment. Smaller decreases in whole-body BMD T score were observed among this group of women at 1 y (P < 0.03) but not at 2 y of treatment. When compared with the placebo, soy isoflavone supplementation had no effect on changes in regional BMD, BMC, T scores, or biochemical markers of bone metabolism.
Conclusion: Daily supplementation with 120 mg soy hypocotyl isoflavones reduces whole-body bone loss but does not slow bone loss at common fracture sites in healthy postmenopausal women. This trial was registered at clinicaltrials.gov as NCT00665860.
Figures


References
-
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95 - PubMed
-
- Lafferty FW, Fiske ME. Postmenopausal estrogen replacement: a long-term cohort study. Am J Med 1994;97:66–77 - PubMed
-
- Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Ann Intern Med 1995;122:9–16 - PubMed
-
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogen and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995;332:1589–93 - PubMed
-
- Risch HA, Howe GR. Menopausal hormone usage and breast cancer in Saskatchewan: a record-linkage cohort study. Am J Epidemiol 1994;139:670–83 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

