Sox9 family members negatively regulate maturation and calcification of chondrocytes through up-regulation of parathyroid hormone-related protein
- PMID: 19759178
- PMCID: PMC2770942
- DOI: 10.1091/mbc.e09-03-0227
Sox9 family members negatively regulate maturation and calcification of chondrocytes through up-regulation of parathyroid hormone-related protein
Abstract
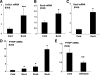
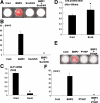
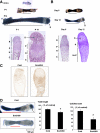
Sox9 is a transcription factor that plays an essential role in chondrogenesis and has been proposed to inhibit the late stages of endochondral ossification. However, the molecular mechanisms underlying the regulation of chondrocyte maturation and calcification by Sox9 remain unknown. In this study, we attempted to clarify roles of Sox9 in the late stages of chondrocyte differentiation. We found that overexpression of Sox9 alone or Sox9 together with Sox5 and Sox6 (Sox5/6/9) inhibited the maturation and calcification of murine primary chondrocytes and up-regulated parathyroid hormone-related protein (PTHrP) expression in primary chondrocytes and the mesenchymal cell line C3H10T1/2. Sox5/6/9 stimulated the early stages of chondrocyte proliferation and development. In contrast, Sox5/6/9 inhibited maturation and calcification of chondrocytes in organ culture. The inhibitory effects of Sox5/6/9 were rescued by treating with anti-PTHrP antibody. Moreover, Sox5/6/9 bound to the promoter region of the PTHrP gene and up-regulated PTHrP gene promoter activity. Interestingly, we also found that the Sox9 family members functionally collaborated with Ihh/Gli2 signaling to regulate PTHrP expression and chondrocyte differentiation. Our results provide novel evidence that Sox9 family members mediate endochondral ossification by up-regulating PTHrP expression in association with Ihh/Gli2 signaling.
Figures




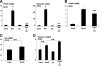
Similar articles
-
Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions.J Biol Chem. 2014 Sep 5;289(36):24898-910. doi: 10.1074/jbc.M114.570507. Epub 2014 Jul 15. J Biol Chem. 2014. PMID: 25028519 Free PMC article.
-
Indian hedgehog signaling promotes chondrocyte differentiation in enchondral ossification in human cervical ossification of the posterior longitudinal ligament.Spine (Phila Pa 1976). 2013 Oct 15;38(22):E1388-96. doi: 10.1097/BRS.0b013e3182a40489. Spine (Phila Pa 1976). 2013. PMID: 23883825
-
The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6.Genes Dev. 2002 Nov 1;16(21):2813-28. doi: 10.1101/gad.1017802. Genes Dev. 2002. PMID: 12414734 Free PMC article.
-
Transcriptional mechanisms of chondrocyte differentiation.Matrix Biol. 2000 Sep;19(5):389-94. doi: 10.1016/s0945-053x(00)00094-9. Matrix Biol. 2000. PMID: 10980415 Review.
-
Interaction of growth factors regulating chondrocyte differentiation in the developing embryo.Osteoarthritis Cartilage. 2001;9 Suppl A:S109-17. Osteoarthritis Cartilage. 2001. PMID: 11680674 Review.
Cited by
-
HDAC5-mediated deacetylation and nuclear localisation of SOX9 is critical for tamoxifen resistance in breast cancer.Br J Cancer. 2019 Dec;121(12):1039-1049. doi: 10.1038/s41416-019-0625-0. Epub 2019 Nov 6. Br J Cancer. 2019. PMID: 31690832 Free PMC article.
-
Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification.J Biol Chem. 2012 Sep 28;287(40):33179-90. doi: 10.1074/jbc.M111.337063. Epub 2012 Aug 6. J Biol Chem. 2012. PMID: 22869368 Free PMC article.
-
Epstein-Barr virus-induced gene 3 commits human mesenchymal stem cells to differentiate into chondrocytes via endoplasmic reticulum stress sensor.PLoS One. 2022 Dec 22;17(12):e0279584. doi: 10.1371/journal.pone.0279584. eCollection 2022. PLoS One. 2022. PMID: 36548354 Free PMC article.
-
SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression.PLoS Genet. 2011 Nov;7(11):e1002356. doi: 10.1371/journal.pgen.1002356. Epub 2011 Nov 3. PLoS Genet. 2011. PMID: 22072985 Free PMC article.
-
SOX9 protein is stabilized by TGF-β and regulates PAPSS2 mRNA expression in chondrocytes.Osteoarthritis Cartilage. 2017 Feb;25(2):332-340. doi: 10.1016/j.joca.2016.10.007. Epub 2016 Oct 13. Osteoarthritis Cartilage. 2017. PMID: 27746378 Free PMC article.
References
-
- Akiyama H., Stadler H. S., Martin J. F., Ishii T. M., Beachy P. A., Nakamura T., de Crombrugghe B. Misexpression of Sox9 in mouse limb bud mesenchyme induces polydactyly and rescues hypodactyly mice. Matrix Biol. 2007;26:224–233. - PubMed
-
- Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod. Rheumatol. 2008;18:213–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

