Language networks in semantic dementia
- PMID: 19759202
- PMCID: PMC2801321
- DOI: 10.1093/brain/awp233
Language networks in semantic dementia
Abstract
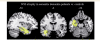
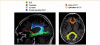
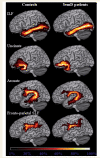
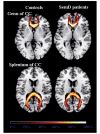
Cognitive deficits in semantic dementia have been attributed to anterior temporal lobe grey matter damage; however, key aspects of the syndrome could be due to altered anatomical connectivity between language pathways involving the temporal lobe. The aim of this study was to investigate the left language-related cerebral pathways in semantic dementia using diffusion tensor imaging-based tractography and to combine the findings with cortical anatomical and functional magnetic resonance imaging data obtained during a reading activation task. The left inferior longitudinal fasciculus, arcuate fasciculus and fronto-parietal superior longitudinal fasciculus were tracked in five semantic dementia patients and eight healthy controls. The left uncinate fasciculus and the genu and splenium of the corpus callosum were also obtained for comparison with previous studies. From each tract, mean diffusivity, fractional anisotropy, as well as parallel and transverse diffusivities were obtained. Diffusion tensor imaging results were related to grey and white matter atrophy volume assessed by voxel-based morphometry and functional magnetic resonance imaging activations during a reading task. Semantic dementia patients had significantly higher mean diffusivity, parallel and transverse in the inferior longitudinal fasciculus. The arcuate and uncinate fasciculi demonstrated significantly higher mean diffusivity, parallel and transverse and significantly lower fractional anisotropy. The fronto-parietal superior longitudinal fasciculus was relatively spared, with a significant difference observed for transverse diffusivity and fractional anisotropy, only. In the corpus callosum, the genu showed lower fractional anisotropy compared with controls, while no difference was found in the splenium. The left parietal cortex did not show significant volume changes on voxel-based morphometry and demonstrated normal functional magnetic resonance imaging activation in response to reading items that stress sublexical phonological processing. This study shows that semantic dementia is associated with anatomical damage to the major superior and inferior temporal white matter connections of the left hemisphere likely involved in semantic and lexical processes, with relative sparing of the fronto-parietal superior longitudinal fasciculus. Fronto-parietal regions connected by this tract were activated normally in the same patients during sublexical reading. These findings contribute to our understanding of the anatomical changes that occur in semantic dementia, and may further help to explain the dissociation between marked single-word and object knowledge deficits, but sparing of phonology and fluency in semantic dementia.
Figures

References
-
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–21. - PubMed
-
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. - PubMed
-
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:529–38. - PubMed
-
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. - PubMed
-
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–32. - PubMed

