Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses
- PMID: 19759314
- PMCID: PMC2775807
- DOI: 10.1523/JNEUROSCI.1542-09.2009
Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses
Abstract
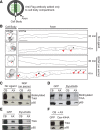
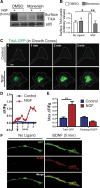
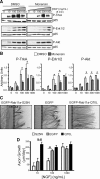
Axonal targeting of trophic receptors is critical for neuronal responses to extracellular developmental cues, yet the underlying trafficking mechanisms remain unclear. Here, we report that tropomyosin-related kinase (Trk) receptors for target-derived neurotrophins are anterogradely trafficked to axons via transcytosis in sympathetic neurons. Using compartmentalized cultures, we show that mature receptors on neuronal soma surfaces are endocytosed and remobilized via Rab11-positive recycling endosomes into axons. Inhibition of dynamin-dependent endocytosis disrupted anterograde transport and localization of TrkA receptors in axons. Anterograde TrkA delivery and exocytosis into axon growth cones is enhanced by nerve growth factor (NGF), acting locally on distal axons. Perturbing endocytic recycling attenuated NGF-dependent signaling and axon growth while enhancing recycling conferred increased neuronal sensitivity to NGF. Our results reveal regulated transcytosis as an unexpected mode of Trk trafficking that serves to rapidly mobilize ready-synthesized receptors to growth cones, thus providing a positive feedback mechanism by which limiting concentrations of target-derived neurotrophins enhance neuronal sensitivity.
Figures



References
-
- Altschuler Y, Hodson C, Milgram SL. The apical compartment: trafficking pathways, regulators and scaffolding proteins. Curr Opin Cell Biol. 2003;15:423–429. - PubMed
-
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. - PubMed
-
- Basu SK, Goldstein JL, Anderson RG, Brown MS. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981;24:493–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
