Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor
- PMID: 19759354
- PMCID: PMC2784726
- DOI: 10.1124/mol.109.058578
Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor
Abstract
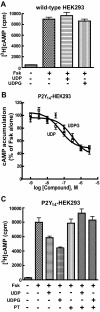
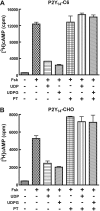
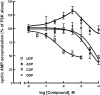
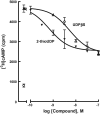
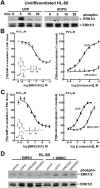
The P2Y14 receptor was initially identified as a G protein-coupled receptor activated by UDP-glucose and other nucleotide sugars. We have developed several cell lines that stably express the human P2Y14 receptor, allowing facile examination of its coupling to native Gi family G proteins and their associated downstream signaling pathways (J Pharmacol Exp Ther 330:162-168, 2009). In the current study, we examined P2Y14 receptor-dependent inhibition of cyclic AMP accumulation in human embryonic kidney (HEK) 293, C6 glioma, and Chinese hamster ovary (CHO) cells stably expressing this receptor. Not only was the human P2Y14 receptor activated by UDP-glucose, but it also was activated by UDP. The apparent efficacies of UDP and UDP-glucose were similar, and the EC50 values (74, 33, and 29 nM) for UDP-dependent activation of the P2Y14 receptor in HEK293, CHO, and C6 glioma cells, respectively, were similar to the EC50 values (323, 132, and 72 nM) observed for UDP-glucose. UDP and UDP-glucose also stimulated extracellular signal-regulated kinase (ERK) 1/2 phosphorylation in P2Y14 receptor-expressing HEK293 cells but not in wild-type HEK293 cells. A series of analogs of UDP were potent P2Y14 receptor agonists, but the naturally occurring nucleoside diphosphates, CDP, GDP, and ADP exhibited agonist potencies over 100-fold less than that observed with UDP. Two UDP analogs were identified that selectively activate the P2Y14 receptor over the UDP-activated P2Y6 receptor, and these molecules stimulated phosphorylation of ERK1/2 in differentiated human HL-60 promyeloleukemia cells, which natively express the P2Y14 receptor but had no effect in wild-type HL-60 cells, which do not express the receptor. We conclude that UDP is an important cognate agonist of the human P2Y14 receptor.
Figures


References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 - PMC - PubMed
-
- Alvarado-Castillo C, Harden TK, Boyer JL. (2005) Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol 67:114–122 - PubMed
-
- Ault AD, Broach JR. (2006) Creation of GPCR-based chemical sensors by directed evolution in yeast. Protein Eng Des Sel 19:1–8 - PubMed
-
- Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

