TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice
- PMID: 19759514
- PMCID: PMC2752063
- DOI: 10.1172/JCI37626
TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice
Abstract
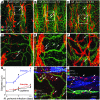
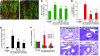
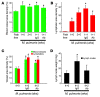
Inflammation is associated with blood vessel and lymphatic vessel proliferation and remodeling. The microvasculature of the mouse trachea provides an ideal opportunity to study this process, as Mycoplasma pulmonis infection of mouse airways induces widespread and sustained vessel remodeling, including enlargement of capillaries into venules and lymphangiogenesis. Although the mediators responsible for these vascular changes in mice have not been identified, VEGF-A is known not to be involved. Here, we sought to determine whether TNF-alpha drives the changes in blood vessels and lymphatics in M. pulmonis-infected mice. The endothelial cells, but not pericytes, of blood vessels, but not lymphatics, were immunoreactive for TNF receptor 1 (TNF-R1) and lymphotoxin B receptors. Most TNF-R2 immunoreactivity was on leukocytes. Infection resulted in a large and sustained increase in TNF-alpha expression, as measured by real-time quantitative RT-PCR, and smaller increases in lymphotoxins and TNF receptors that preceded vessel remodeling. Substantially less vessel remodeling and lymphangiogenesis occurred when TNF-alpha signaling was inhibited by a blocking antibody or was silenced in Tnfr1-/- mice. When administered after infection was established, the TNF-alpha-specific antibody slowed but did not reverse blood vessel remodeling and lymphangiogenesis. The action of TNF-alpha on blood vessels is probably mediated through direct effects on endothelial cells, but its effects on lymphangiogenesis may require inflammatory mediators from recruited leukocytes. We conclude that TNF-alpha is a strong candidate for a mediator that drives blood vessel remodeling and lymphangiogenesis in inflammation.
Figures




References
-
- Aurora A.B., et al. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J. Immunol. 2005;175:6319–6326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

