Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer
- PMID: 19759520
- PMCID: PMC2752070
- DOI: 10.1172/JCI38746
Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer
Abstract
EGFR is a major anticancer drug target in human epithelial tumors. One effective class of agents is the tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib. These drugs induce dramatic responses in individuals with lung adenocarcinomas characterized by mutations in exons encoding the EGFR tyrosine kinase domain, but disease progression invariably occurs. A major reason for such acquired resistance is the outgrowth of tumor cells with additional TKI-resistant EGFR mutations. Here we used relevant transgenic mouse lung tumor models to evaluate strategies to overcome the most common EGFR TKI resistance mutation, T790M. We treated mice bearing tumors harboring EGFR mutations with a variety of anticancer agents, including a new irreversible EGFR TKI that is under development (BIBW-2992) and the EGFR-specific antibody cetuximab. Surprisingly, we found that only the combination of both agents together induced dramatic shrinkage of erlotinib-resistant tumors harboring the T790M mutation, because together they efficiently depleted both phosphorylated and total EGFR. We suggest that these studies have immediate therapeutic implications for lung cancer patients, as dual targeting with cetuximab and a second-generation EGFR TKI may be an effective strategy to overcome T790M-mediated drug resistance. Moreover, this approach could serve as an important model for targeting other receptor tyrosine kinases activated in human cancers.
Figures

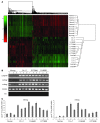
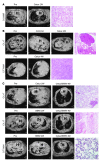

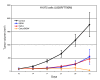

References
Publication types
MeSH terms
Substances
Grants and funding
- K08 CA097980/CA/NCI NIH HHS/United States
- K08-CA097980/CA/NCI NIH HHS/United States
- R01-CA120247/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P30-CA68485/CA/NCI NIH HHS/United States
- R01 CA121210/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P30-CA008748/CA/NCI NIH HHS/United States
- R01-CA121210/CA/NCI NIH HHS/United States
- CA90949/CA/NCI NIH HHS/United States
- R01 CA120247/CA/NCI NIH HHS/United States
- P50 CA090949/CA/NCI NIH HHS/United States
- K99 CA131488/CA/NCI NIH HHS/United States
- K99-CA131488/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

