PSSA-2, a membrane-spanning phosphoprotein of Trypanosoma brucei, is required for efficient maturation of infection
- PMID: 19759911
- PMCID: PMC2739429
- DOI: 10.1371/journal.pone.0007074
PSSA-2, a membrane-spanning phosphoprotein of Trypanosoma brucei, is required for efficient maturation of infection
Abstract
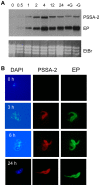
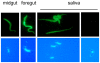
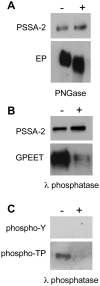
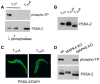
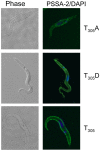
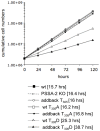
The coat of Trypanosoma brucei consists mainly of glycosylphosphatidylinositol-anchored proteins that are present in several million copies and are characteristic of defined stages of the life cycle. While these major components of the coats of bloodstream forms and procyclic (insect midgut) forms are well characterised, very little is known about less abundant stage-regulated surface proteins and their roles in infection and transmission. By creating epitope-tagged versions of procyclic-specific surface antigen 2 (PSSA-2) we demonstrated that it is a membrane-spanning protein that is expressed by several different life cycle stages in tsetse flies, but not by parasites in the mammalian bloodstream. In common with other membrane-spanning proteins in T. brucei, PSSA-2 requires its cytoplasmic domain in order to exit the endoplasmic reticulum. Correct localisation of PSSA-2 requires phosphorylation of a cytoplasmic threonine residue (T(305)), a modification that depends on the presence of TbMAPK4. Mutation of T(305) to alanine (T(305)A) has no effect on the localisation of the protein in cells that express wild type PSSA-2. In contrast, this protein is largely intracellular when expressed in a null mutant background. A variant with a T(305)D mutation gives strong surface expression in both the wild type and null mutant, but slows growth of the cells, suggesting that it may function as a dominant negative mutant. The PSSA-2 null mutant exhibits no perceptible phenotype in culture and is fully competent at establishing midgut infections in tsetse, but is defective in colonising the salivary glands and the production of infectious metacyclic forms. Given the protein's structure and the effects of mutation of T(305) on proliferation and localisation, we postulate that PSSA-2 might sense and transmit signals that contribute to the parasite's decision to divide, differentiate or migrate.
Conflict of interest statement
Figures


References
-
- Urwyler S, Studer E, Renggli CK, Roditi I. A family of stage-specific alanine-rich proteins on the surface of epimastigote forms of Trypanosoma brucei. Mol Microbiol. 2007;63:218–228. - PubMed
-
- Hajduk SL. Antigenic variation during the developmental cycle of Trypanosoma brucei. J Protozool. 1984;31:41–47. - PubMed
-
- Bayne RA, Kilbride EA, Lainson FA, Tetley L, Barry JD. A major surface antigen of procyclic stage Trypanosoma congolense. Mol Biochem Parasitol. 1993;61:295–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

