Biophysical characterization of the iron in mitochondria from Atm1p-depleted Saccharomyces cerevisiae
- PMID: 19761223
- PMCID: PMC2758324
- DOI: 10.1021/bi901110n
Biophysical characterization of the iron in mitochondria from Atm1p-depleted Saccharomyces cerevisiae
Abstract
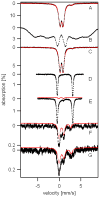
Atm1p is an ABC transporter localized in the mitochondrial inner membrane; it functions to export an unknown species into the cytosol and is involved in cellular iron metabolism. Depletion or deletion of Atm1p causes Fe accumulation in mitochondria and a defect in cytosolic Fe/S cluster assembly but reportedly not a defect in mitochondrial Fe/S cluster assembly. In this study the nature of the accumulated Fe was examined using Mossbauer spectroscopy, EPR, electronic absorption spectroscopy, X-ray absorption spectroscopy, and electron microscopy. The Fe that accumulated in aerobically grown cells was in the form of iron(III) phosphate nanoparticles similar to that which accumulates in yeast frataxin Yfh1p-deleted or yeast ferredoxin Yah1p-depleted cells. Relative to WT mitochondria, Fe/S cluster and heme levels in Atm1p-depleted mitochondria from aerobic cells were significantly diminished. Atm1p depletion also caused a buildup of nonheme Fe(II) ions in the mitochondria and an increase in oxidative damage. Atm1p-depleted mitochondria isolated from anaerobically grown cells exhibited WT levels of Fe/S clusters and hemes, and they did not hyperaccumulate Fe. Atm1p-depleted cells lacked Leu1p activity, regardless of whether they were grown aerobically or anaerobically. These results indicate that Atm1p does not participate in mitochondrial Fe/S cluster assembly and that the species exported by Atm1p is required for cytosolic Fe/S cluster assembly. The Fe/S cluster defect and the Fe-accumulation phenotype, resulting from the depletion of Atm1p in aerobic cells (but not in anaerobic cells), may be secondary effects that are observed only when cells are exposed to oxygen during growth. Reactive oxygen species generated under these conditions might degrade iron-sulfur clusters and lower heme levels in the organelle.
Figures










References
-
- Labbebois R. The Ferrochelatase from Saccharomyces Cerevisiae - Sequence, Disruption, and Expression of Its Structural Gene Hem15. J Biol Chem. 1990;265:7278–7283. - PubMed
-
- Lill R, Mühlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: Mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. - PubMed
-
- Kuhnke G, Neumann K, Mühlenhoff U, Lill R. Stimulation of the ATPase activity of the yeast mitochondrial ABC transporter Atm1p by thiol compounds. Mol Membr Biol. 2006;23:173–184. - PubMed
-
- Hausmann A, Samans B, Lill R, Mühlenhoff U. Cellular and mitochondrial remodeling upon defects in iron-sulfur protein biogenesis. J Biol Chem. 2008;283:8318–8330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

