Selective targeting of ER exit sites supports axon development
- PMID: 19761544
- PMCID: PMC2763039
- DOI: 10.1111/j.1600-0854.2009.00974.x
Selective targeting of ER exit sites supports axon development
Abstract
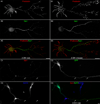
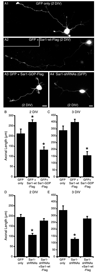
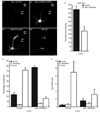
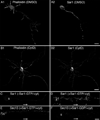
During neuron development, the biosynthetic needs of the axon initially outweigh those of dendrites. However, although a localized role for the early secretory pathway in dendrite development has been observed, such a role in axon growth remains undefined. We therefore studied the localization of Sar1, a small GTPase that controls ER export, during early stages of neuronal development that are characterized by selective and robust axon growth. At these early stages, Sar1 was selectively targeted to the axon where it gradually concentrated within varicosities in which additional proteins that function in the early secretory pathway were detected. Sar1 targeting to the axon followed axon specification and was dependent on localized actin instability. Changes in Sar1 expression levels at these early development stages modulated axon growth. Specifically, reduced expression of Sar1, which was initially only detectable in the axon, correlated with reduced axon growth, where as overexpression of Sar1 supported the growth of longer axons. In support of the former finding, expression of dominant negative Sar1 inhibited axon growth. Thus, as observed in lower organisms, mammalian cells use temporal and spatial regulation of endoplasmic reticulum exit site (ERES) to address developmental biosynthetic demands. Furthermore, axons, such as dendrites, rely on ERES targeting and assembly for growth.
Figures



References
-
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5(4):287–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

