Anti-proliferative activity of silver nanoparticles
- PMID: 19761582
- PMCID: PMC2759918
- DOI: 10.1186/1471-2121-10-65
Anti-proliferative activity of silver nanoparticles
Abstract
Background: Nanoparticles possess exceptional physical and chemical properties which led to rapid commercialisation. Silver nanoparticles (Ag-np) are among the most commercialized nanoparticles due to their antimicrobial potential. Ag-np based cosmetics, therapeutic agents and household products are in wide use, which raised a public concern regarding their safety associated with human and environmental use. No safety regulations are in practice for the use of these nanomaterials. The interactions of nanomaterials with cells, uptake mechanisms, distribution, excretion, toxicological endpoints and mechanism of action remain unanswered.
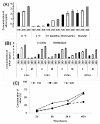
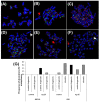
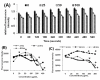
Results: Normal human lung fibroblasts (IMR-90) and human glioblastoma cells (U251) were exposed to different doses of Ag-nps in vitro. Uptake of Ag-nps occurred mainly through endocytosis (clathrin mediated process and macropinocytosis), accompanied by a time dependent increase in exocytosis rate. The electron micrographs revealed a uniform intracellular distribution of Ag-np both in cytoplasm and nucleus. Ag-np treated cells exhibited chromosome instability and mitotic arrest in human cells. There was efficient recovery from arrest in normal human fibroblasts whereas the cancer cells ceased to proliferate. Toxicity of Ag-np is mediated through intracellular calcium (Ca2+) transients along with significant alterations in cell morphology and spreading and surface ruffling. Down regulation of major actin binding protein, filamin was observed after Ag-np exposure. Ag-np induced stress resulted in the up regulation of metallothionein and heme oxygenase -1 genes.
Conclusion: Here, we demonstrate that uptake of Ag-np occurs mainly through clathrin mediated endocytosis and macropinocytosis. Our results suggest that cancer cells are susceptible to damage with lack of recovery from Ag-np-induced stress. Ag-np is found to be acting through intracellular calcium transients and chromosomal aberrations, either directly or through activation of catabolic enzymes. The signalling cascades are believed to play key roles in cytoskeleton deformations and ultimately to inhibit cell proliferation.
Figures





References
-
- Kumar C. Nanomaterials- Toxicity, health and Environmental issues. Wiley- VCH Verlag GmbH & Co, Weinheim; 2006.
-
- Zhao Y, Nalwa H. Nanotoxicology - Interactions of Nanomaterials with Biological Systems. California, USA: American Scienfici Publishers; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

