Akt-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development
- PMID: 19762425
- PMCID: PMC2752394
- DOI: 10.1242/dev.037010
Akt-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development
Abstract
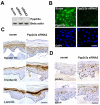
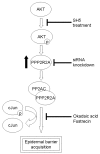
Acquisition of epidermal barrier function occurs late in mouse gestation. Several days before birth a wave of barrier acquisition sweeps across murine fetal skin, converging on dorsal and ventral midlines. We investigated the molecular pathways active during epidermal barrier formation. Akt signaling increased as the barrier wave crossed epidermis and Jun was transiently dephosphorylated. Inhibitor experiments on embryonic explants showed that the dephosphorylation of Jun was dependent on both Akt and protein phosphatase 2A (Pp2a). Inhibition of Pp2a and Akt signaling also caused defects in epidermal barrier formation. These data are compatible with a model for developmental barrier acquisition mediated by Pp2a regulation of Jun dephosphorylation, downstream of Akt signaling. Support for this model was provided by siRNA-mediated knockdown of Ppp2r2a (Pr55alpha or B55alpha), a regulatory subunit of Pp2a expressed in an Akt-dependent manner in epidermis during barrier formation. Ppp2r2a reduction caused significant increase in Jun phosphorylation and interfered with the acquisition of barrier function, with barrier acquisition being restored by inhibition of Jun phosphorylation. Our data provide strong evidence that Ppp2r2a is a regulatory subunit of Pp2a that targets this phosphatase to Jun, and that Pp2a action is necessary for barrier formation. We therefore describe a novel Akt-dependent Pp2a activity that acts at least partly through Jun to affect initial barrier formation during late embryonic epidermal development.
Figures







Similar articles
-
The protein phosphatase 2A regulatory subunit Ppp2r2a is required for Connexin-43 dephosphorlyation during epidermal barrier acquisition.Exp Dermatol. 2013 Nov;22(11):754-6. doi: 10.1111/exd.12234. Exp Dermatol. 2013. PMID: 24433183
-
Rab3Gap1 mediates exocytosis of Claudin-1 and tight junction formation during epidermal barrier acquisition.Dev Biol. 2013 Aug 15;380(2):274-85. doi: 10.1016/j.ydbio.2013.04.034. Epub 2013 May 16. Dev Biol. 2013. PMID: 23685254 Free PMC article.
-
Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt.J Biol Chem. 2008 Jan 25;283(4):1882-92. doi: 10.1074/jbc.M709585200. Epub 2007 Nov 27. J Biol Chem. 2008. PMID: 18042541
-
Protein kinases involved in epidermal barrier formation: The AKT family and other animals.Exp Dermatol. 2018 Aug;27(8):892-900. doi: 10.1111/exd.13696. Exp Dermatol. 2018. PMID: 29845670 Review.
-
Role of mTOR Signaling Cascade in Epidermal Morphogenesis and Skin Barrier Formation.Biology (Basel). 2022 Jun 19;11(6):931. doi: 10.3390/biology11060931. Biology (Basel). 2022. PMID: 35741452 Free PMC article. Review.
Cited by
-
Transcriptomics analyses reveal the effects of Pentagamaboronon-0-ol on PI3K/Akt and cell cycle of HER2+ breast cancer cells.Saudi Pharm J. 2023 Dec;31(12):101847. doi: 10.1016/j.jsps.2023.101847. Epub 2023 Oct 27. Saudi Pharm J. 2023. PMID: 38028209 Free PMC article.
-
Protein Phosphatase 2A: More Than a Passenger in the Regulation of Epithelial Cell-Cell Junctions.Front Cell Dev Biol. 2019 Mar 6;7:30. doi: 10.3389/fcell.2019.00030. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 30895176 Free PMC article. Review.
-
Palatogenesis and cutaneous repair: A two-headed coin.Dev Dyn. 2015 Mar;244(3):289-310. doi: 10.1002/dvdy.24224. Epub 2014 Nov 25. Dev Dyn. 2015. PMID: 25370680 Free PMC article. Review.
-
Upregulated SET Promotes Cell Survival Through Activating Akt/NF-κB Signal in Colorectal Carcinoma.Cancer Manag Res. 2020 Jun 19;12:4735-4745. doi: 10.2147/CMAR.S255930. eCollection 2020. Cancer Manag Res. 2020. PMID: 32606964 Free PMC article.
-
PP2A-B55alpha controls keratinocyte adhesion through dephosphorylation of the Desmoplakin C-terminus.Sci Rep. 2023 Aug 5;13(1):12720. doi: 10.1038/s41598-023-37874-8. Sci Rep. 2023. PMID: 37543698 Free PMC article.
References
-
- Avdi, N. J., Malcolm, K. C., Nick, J. A. and Worthen, G. S. (2002). A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J. Biol. Chem. 277, 40687-40696. - PubMed
-
- Backman, S. A., Ghazarian, D., So, K., Sanchez, O., Wagner, K. U., Hennighausen, L., Suzuki, A., Tsao, M. S., Chapman, W. B., Stambolic, V. et al. (2004). Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc. Natl. Acad. Sci. USA 101, 1725-1730. - PMC - PubMed
-
- Baden, H. P. and Kubilus, J. (1983). The growth and differentiation of cultured newborn rat keratinocytes. J. Invest. Dermatol. 80, 124-130. - PubMed
-
- Basset-Seguin, N., Escot, C., Moles, J. P., Blanchard, J. M., Kerai, C. and Guilhou, J. J. (1991). C-fos and c-jun proto-oncogene expression is decreased in psoriasis: an in situ quantitative analysis. J. Invest. Dermatol. 97, 672-678. - PubMed
-
- Binetruy, B., Smeal, T. and Karin, M. (1991). Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature 351, 122-127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

