Auditory midbrain implant: a review
- PMID: 19762428
- PMCID: PMC4111439
- DOI: 10.1177/1084713809348372
Auditory midbrain implant: a review
Abstract
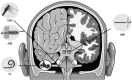
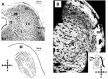
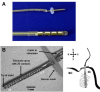
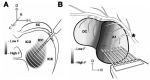
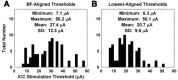
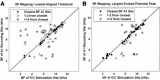
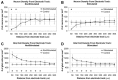
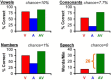
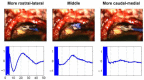
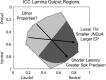
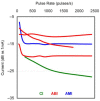
The auditory midbrain implant (AMI) is a new hearing prosthesis designed for stimulation of the inferior colliculus in deaf patients who cannot sufficiently benefit from cochlear implants. The authors have begun clinical trials in which five patients have been implanted with a single shank AMI array (20 electrodes). The goal of this review is to summarize the development and research that has led to the translation of the AMI from a concept into the first patients. This study presents the rationale and design concept for the AMI as well a summary of the animal safety and feasibility studies that were required for clinical approval. The authors also present the initial surgical, psychophysical, and speech results from the first three implanted patients. Overall, the results have been encouraging in terms of the safety and functionality of the implant. All patients obtain improvements in hearing capabilities on a daily basis. However, performance varies dramatically across patients depending on the implant location within the midbrain with the best performer still not able to achieve open set speech perception without lip-reading cues. Stimulation of the auditory midbrain provides a wide range of level, spectral, and temporal cues, all of which are important for speech understanding, but they do not appear to sufficiently fuse together to enable open set speech perception with the currently used stimulation strategies. Finally, several issues and hypotheses for why current patients obtain limited speech perception along with several feasible solutions for improving AMI implementation are presented.
Figures










References
-
- Adams J. S., Hasenstab M. S., Pippin G. W., Sismanis A. (2004). Telephone use and understanding in patients with cochlear implants. Ear, Nose, & Throat Journal, 83, 6, 99–100, 102–103 - PubMed
-
- Ammirati M., Bernardo A., Musumeci A., Bricolo A. (2002). Comparison of different infratentorial-supracerebellar approaches to the posterior and middle incisural space: A cadaveric study. Journal of Neurosurgery, 97, 922–928 - PubMed
-
- Andersen R. A., Roth G. L., Aitkin L. M., Merzenich M. M. (1980). The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. Journal of Comparative Neurology, 194, 649–662 - PubMed
-
- Anderson D. J. (2008). Penetrating multichannel stimulation and recording electrodes in auditory prosthesis research. Hearing Research, 242, 31–41 - PubMed
-
- Andreev A. M., Gersuni G. V., Volokhov A. A. (1935). On the electrical excitability of the human ear: On the effect of alternating currents on the affected auditory apparatus. Journal of Physiology USSR, 18, 250–265
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
