Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance
- PMID: 19762485
- PMCID: PMC4081374
- DOI: 10.1182/blood-2009-07-233718
Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance
Abstract
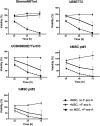
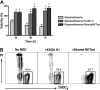
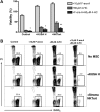
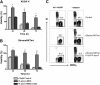
Marrow stromal cells (MSCs) provide important survival and drug resistance signals to chronic lymphocytic leukemia (CLL) cells, but current models to analyze CLL-MSC interactions are heterogeneous. Therefore, we tested different human and murine MSC lines and primary human MSCs for their ability to protect CLL cells from spontaneous and drug-induced apoptosis. Our results show that both human and murine MSCs are equally effective in protecting CLL cells from fludarabine-induced apoptosis. This protective effect was sustained over a wide range of CLL-MSC ratios (5:1 to 100:1), and the levels of protection were reproducible in 4 different laboratories. Human and murine MSCs also protected CLL cells from dexamethasone- and cyclophosphamide-induced apoptosis. This protection required cell-cell contact and was virtually absent when CLL cells were separated from the MSCs by micropore filters. Furthermore, MSCs maintained Mcl-1 and protected CLL cells from spontaneous and fludarabine-induced Mcl-1 and PARP cleavage. Collectively, these studies define common denominators for CLL cocultures with MSCs. They also provide a reliable, validated tool for future investigations into the mechanism of MSC-CLL cross talk and for drug testing in a more relevant fashion than the commonly used suspension cultures.
Figures



References
-
- Rawstron AC, Kennedy B, Evans PA, et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood. 2001;98(1):29–35. - PubMed
-
- Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91(7):2387–2396. - PubMed
-
- Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43(3):461–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

