Proliferation and migration of label-retaining cells of the kidney papilla
- PMID: 19762493
- PMCID: PMC2799170
- DOI: 10.1681/ASN.2008111203
Proliferation and migration of label-retaining cells of the kidney papilla
Abstract
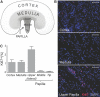
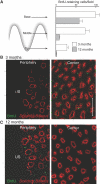
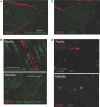
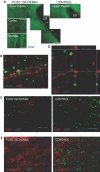
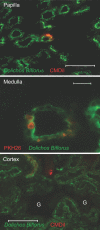
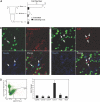
The kidney papilla contains a population of cells with several characteristics of adult stem cells, including the retention of proliferation markers during long chase periods (i.e., they are label-retaining cells [LRCs]). To determine whether the papillary LRCs generate new cells in the normal adult kidney, we examined cell proliferation throughout the kidney and found that the upper papilla is a site of enhanced cell cycling. Using genetically modified mice that conditionally expressed green fluorescence protein fused to histone 2B, we observed that the LRCs of the papilla proliferated only in its upper part, where they associate with "chains" of cycling cells. The papillary LRCs decreased in number with age, suggesting that the cells migrated to the upper papilla before entering the cell cycle. To test this directly, we marked papillary cells with vital dyes in vivo and found that some cells in the kidney papilla, including LRCs, migrated toward other parts of the kidney. Acute kidney injury enhanced both cell migration and proliferation. These results suggest that during normal homeostasis, LRCs of the kidney papilla (or their immediate progeny) migrate to the upper papilla and form a compartment of rapidly proliferating cells, which may play a role in repair after ischemic injury.
Figures

Comment in
-
Slow-cycling cells in renal papilla: stem cells awaken?J Am Soc Nephrol. 2009 Nov;20(11):2277-9. doi: 10.1681/ASN.2009090911. J Am Soc Nephrol. 2009. PMID: 19880716 No abstract available.
-
The aging kidney phenotype and systemically derived stem cells.J Am Soc Nephrol. 2011 Nov;22(11):1958-60. doi: 10.1681/ASN.2011090946. Epub 2011 Oct 13. J Am Soc Nephrol. 2011. PMID: 21997395 No abstract available.
References
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P: Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 658–659, 2003 - PubMed
-
- Petersen BE, Zajac VF, Michalopoulos GK: Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology 27: 1030–1038, 1998 - PubMed
-
- Mothe AJ, Tator CH: Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult bat. Neuroscience 131: 177–187, 2005 - PubMed
-
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM: Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102: 451–461, 2000 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

