Six-month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection
- PMID: 19762495
- PMCID: PMC2799179
- DOI: 10.1681/ASN.2008111166
Six-month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection
Abstract
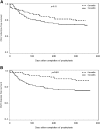
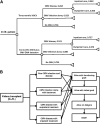
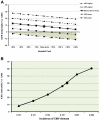
The risk of late-onset cytomegalovirus (CMV) infection remains a concern in seronegative kidney and/or pancreas transplant recipients of seropositive organs despite the use of antiviral prophylaxis. The optimal duration of prophylaxis is unknown. We studied the cost effectiveness of 6- versus 3-mo prophylaxis with valganciclovir. A total of 222 seronegative recipients of seropositive kidney and/or pancreas transplants received valganciclovir prophylaxis for either 3 or 6 mo during two consecutive time periods. We assessed the incidence of CMV infection and disease 12 mo after completion of prophylaxis and performed cost-effectiveness analyses. The overall incidence of CMV infection and disease was 26.7% and 24.4% in the 3-mo group and 20.9% and 12.1% in the 6-mo group, respectively. Six-month prophylaxis was associated with a statistically significant reduction in risk for CMV disease (HR, 0.35; 95% CI, 0.17 to 0.72), but not infection (HR, 0.65; 95% CI, 0.37 to 1.14). Cost-effectiveness analyses showed that 6-mo prophylaxis combined with a one-time viremia determination at the end of the prophylaxis period incurred an incremental cost of $34,362 and $16,215 per case of infection and disease avoided, respectively, and $8,304 per one quality adjusted life-year gained. Sensitivity analyses supported the cost effectiveness of 6-mo prophylaxis over a wide range of valganciclovir and hospital costs, as well as variation in the incidence of CMV disease. In summary, 6-mo prophylaxis with valganciclovir combined with a one-time determination of viremia is cost effective in reducing CMV infection and disease in seronegative recipients of seropositive kidney and/or pancreas transplants.
Figures
References
-
- Rubin RH: Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis 12[Suppl 7]: S754–S766, 1990 - PubMed
-
- Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD: Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4: 611–620, 2004 - PubMed
-
- Schnitzler MA, Lowell JA, Hardinger KL, Boxerman SB, Bailey TC, Brennan DC: The association of cytomegalovirus sero-pairing with outcomes and costs following cadaveric renal transplantation prior to the introduction of oral ganciclovir CMV prophylaxis. Am J Transplant 3: 445–451, 2003 - PubMed
-
- Akalin E, Sehgal V, Ames S, Hossain S, Daly L, Barbara M, Bromberg JS: Cytomegalovirus disease in high-risk transplant recipients despite ganciclovir or valganciclovir prophylaxis. Am J Transplant 3: 731–735, 2003 - PubMed
-
- Johnson PC, Lewis RM, Golden DL, Oefinger PE, Van Buren CT, Kerman RH, Kahan BD: The impact of cytomegalovirus infection on seronegative recipients of seropositive donor kidneys versus seropositive recipients treated with cyclosporine-prednisone immunosuppression. Transplantation 45: 116–121, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

