Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2
- PMID: 19762917
- PMCID: PMC2781491
- DOI: 10.1074/jbc.M109.021238
Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2
Abstract
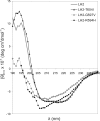
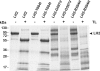
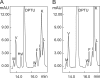
Bruck syndrome is a rare autosomal recessive connective tissue disorder characterized by fragile bones, joint contractures, scoliosis, and osteoporosis. The telopeptides of bone collagen I are underhydroxylated in these patients, leading to abnormal collagen cross-linking. Three point mutations in lysyl hydroxylase (LH) 2, the enzyme responsible for the hydroxylation of collagen telopeptides, have been identified in Bruck syndrome. As none of them affects the residues known to be critical for LH activity, we studied their consequences at the molecular level by analyzing the folding and catalytic properties of the corresponding mutant recombinant polypeptides. Folding and oligomerization of the R594H and G597V mutants were abnormal, and their activity was reduced by >95% relative to the wild type. The T604I mutation did not affect the folding properties, although the mutant retained only approximately 8% activity under standard assay conditions. As the reduced activity was caused by a 10-fold increase in the K(m) for 2-oxoglutarate, the mutation interferes with binding of this cosubstrate. In the presence of a saturating 2-oxoglutarate concentration, the activity of the T604I mutant was approximately 30% of that of the wild type. However, the T604I mutant did not generate detectable amounts of hydroxylysine in the N-terminal telopeptide of a recombinant procollagen I chain when coexpressed in insect cells. The low activity of the mutant LH2 polypeptides is in accordance with the markedly reduced extent of collagen telopeptide hydroxylation in Bruck syndrome, with consequent changes in the cross-linking of collagen fibrils and severe abnormalities in the skeletal structures.
Figures



References
-
- Kivirikko K. I., Pihlajaniemi T. (1998) Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325–398 - PubMed
-
- Myllyharju J., Kivirikko K. I. (2004) Trends Genet. 20, 34–45 - PubMed
-
- Myllyharju J. (2005) Top. Curr. Chem. 247, 115–147
-
- Valtavaara M., Papponen H., Pirttilä A. M., Hiltunen K., Helander H., Myllylä R. (1997) J. Biol. Chem. 272, 6831–6834 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

