NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain
- PMID: 19763089
- PMCID: PMC2760117
- DOI: 10.1038/emboj.2009.241
NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain
Abstract
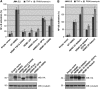
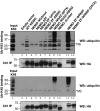
An important property of NEMO, the core element of the IKK complex involved in NF-kappaB activation, resides in its ability to specifically recognize poly-ubiquitin chains. A small domain called NOA/UBAN has been suggested to be responsible for this property. We recently demonstrated that the C-terminal Zinc Finger (ZF) of NEMO is also able to bind ubiquitin. We show here by ZF swapping and mutagenesis that this represents its only function. While neither NOA nor ZF shows any preference for K63-linked chains, we demonstrate that together they form a bipartite high-affinity K63-specific ubiquitin-binding domain. A similar domain can be found in two other proteins, Optineurin and ABIN2, and can be freely exchanged with that of NEMO without interfering with its activity. This suggests that the main function of the C-terminal half of NEMO is to specifically bind K63-linked poly-ubiquitin chains. We also demonstrate that the recently described binding of NEMO to linear poly-ubiquitin chains is dependent on the NOA alone and does not require the presence of the ZF.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Bagnéris C, Ageichik AV, Cronin N, Wallace B, Collins M, Boshoff C, Waksman G, Barrett T (2008) Crystal structure of a vFlip–IKKgamma complex: insights into viral activation of the IKK signalosome. Mol Cell 30: 620–631 - PubMed
-
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310: 1821–1824 - PubMed
-
- Bish RA, Fregoso OI, Piccini A, Myers MP (2008) Conjugation of complex polyubiquitin chains to WRNIP1. J Proteome Res 7: 3481–3489 - PubMed
-
- Bish RA, Myers MP (2007) Werner helicase-interacting protein 1 binds polyubiquitin via its zinc finger domain. J Biol Chem 282: 23184–23193 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

