Multiple organ system defects and transcriptional dysregulation in the Nipbl(+/-) mouse, a model of Cornelia de Lange Syndrome
- PMID: 19763162
- PMCID: PMC2730539
- DOI: 10.1371/journal.pgen.1000650
Multiple organ system defects and transcriptional dysregulation in the Nipbl(+/-) mouse, a model of Cornelia de Lange Syndrome
Abstract
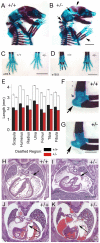
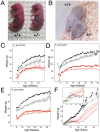
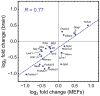
Cornelia de Lange Syndrome (CdLS) is a multi-organ system birth defects disorder linked, in at least half of cases, to heterozygous mutations in the NIPBL gene. In animals and fungi, orthologs of NIPBL regulate cohesin, a complex of proteins that is essential for chromosome cohesion and is also implicated in DNA repair and transcriptional regulation. Mice heterozygous for a gene-trap mutation in Nipbl were produced and exhibited defects characteristic of CdLS, including small size, craniofacial anomalies, microbrachycephaly, heart defects, hearing abnormalities, delayed bone maturation, reduced body fat, behavioral disturbances, and high mortality (75-80%) during the first weeks of life. These phenotypes arose despite a decrease in Nipbl transcript levels of only approximately 30%, implying extreme sensitivity of development to small changes in Nipbl activity. Gene expression profiling demonstrated that Nipbl deficiency leads to modest but significant transcriptional dysregulation of many genes. Expression changes at the protocadherin beta (Pcdhb) locus, as well as at other loci, support the view that NIPBL influences long-range chromosomal regulatory interactions. In addition, evidence is presented that reduced expression of genes involved in adipogenic differentiation may underlie the low amounts of body fat observed both in Nipbl+/- mice and in individuals with CdLS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


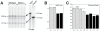

Similar articles
-
Multifactorial origins of heart and gut defects in nipbl-deficient zebrafish, a model of Cornelia de Lange Syndrome.PLoS Biol. 2011 Oct;9(10):e1001181. doi: 10.1371/journal.pbio.1001181. Epub 2011 Oct 25. PLoS Biol. 2011. PMID: 22039349 Free PMC article.
-
Reduction of Nipbl impairs cohesin loading locally and affects transcription but not cohesion-dependent functions in a mouse model of Cornelia de Lange Syndrome.Biochim Biophys Acta. 2013 Dec;1832(12):2097-102. doi: 10.1016/j.bbadis.2013.07.020. Epub 2013 Aug 3. Biochim Biophys Acta. 2013. PMID: 23920377 Free PMC article.
-
The effect of Nipped-B-like (Nipbl) haploinsufficiency on genome-wide cohesin binding and target gene expression: modeling Cornelia de Lange syndrome.Clin Epigenetics. 2017 Aug 25;9:89. doi: 10.1186/s13148-017-0391-x. eCollection 2017. Clin Epigenetics. 2017. PMID: 28855971 Free PMC article.
-
Using mouse and zebrafish models to understand the etiology of developmental defects in Cornelia de Lange Syndrome.Am J Med Genet C Semin Med Genet. 2016 Jun;172(2):138-45. doi: 10.1002/ajmg.c.31484. Epub 2016 Apr 27. Am J Med Genet C Semin Med Genet. 2016. PMID: 27120001 Free PMC article. Review.
-
Cornelia de Lange syndrome.Adv Exp Med Biol. 2010;685:111-23. Adv Exp Med Biol. 2010. PMID: 20687500 Review.
Cited by
-
The contribution of cohesin-SA1 to gene expression and chromatin architecture in two murine tissues.Nucleic Acids Res. 2015 Mar 31;43(6):3056-67. doi: 10.1093/nar/gkv144. Epub 2015 Mar 3. Nucleic Acids Res. 2015. PMID: 25735743 Free PMC article.
-
Genetically induced redox stress occurs in a yeast model for Roberts syndrome.G3 (Bethesda). 2022 Feb 4;12(2):jkab426. doi: 10.1093/g3journal/jkab426. G3 (Bethesda). 2022. PMID: 34897432 Free PMC article.
-
The Drosophila enhancer of split gene complex: architecture and coordinate regulation by notch, cohesin, and polycomb group proteins.G3 (Bethesda). 2013 Oct 3;3(10):1785-94. doi: 10.1534/g3.113.007534. G3 (Bethesda). 2013. PMID: 23979932 Free PMC article.
-
Inactivation of the budding yeast cohesin loader Scc2 alters gene expression both globally and in response to a single DNA double strand break.Cell Cycle. 2014;13(23):3645-58. doi: 10.4161/15384101.2014.964108. Cell Cycle. 2014. PMID: 25483075 Free PMC article.
-
Three-dimensional genome structure and function.MedComm (2020). 2023 Jul 8;4(4):e326. doi: 10.1002/mco2.326. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37426677 Free PMC article. Review.
References
-
- de Lange C. Sur un type nouveau de degeneration (typus Amstelodamensis). Arch Med Enfants. 1933;36:713–719.
-
- Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993;47:940–946. - PubMed
-
- Ireland M, Donnai D, Burn J. Brachmann-de Lange syndrome. Delineation of the clinical phenotype. Am J Med Genet. 1993;47:959–964. - PubMed
-
- Braddock SR, Lachman RS, Stoppenhagen CC, Carey JC, Ireland M, et al. Radiological features in Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:1006–1013. - PubMed
-
- Mehta AV, Ambalavanan SK. Occurrence of congenital heart disease in children with Brachmann-de Lange syndrome. Am J Med Genet. 1997;71:434–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

