RAD50 is required for efficient initiation of resection and recombinational repair at random, gamma-induced double-strand break ends
- PMID: 19763170
- PMCID: PMC2734177
- DOI: 10.1371/journal.pgen.1000656
RAD50 is required for efficient initiation of resection and recombinational repair at random, gamma-induced double-strand break ends
Abstract
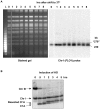
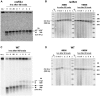
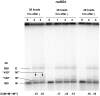
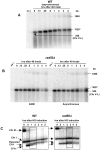
Resection of DNA double-strand break (DSB) ends is generally considered a critical determinant in pathways of DSB repair and genome stability. Unlike for enzymatically induced site-specific DSBs, little is known about processing of random "dirty-ended" DSBs created by DNA damaging agents such as ionizing radiation. Here we present a novel system for monitoring early events in the repair of random DSBs, based on our finding that single-strand tails generated by resection at the ends of large molecules in budding yeast decreases mobility during pulsed field gel electrophoresis (PFGE). We utilized this "PFGE-shift" to follow the fate of both ends of linear molecules generated by a single random DSB in circular chromosomes. Within 10 min after gamma-irradiation of G2/M arrested WT cells, there is a near-synchronous PFGE-shift of the linearized circular molecules, corresponding to resection of a few hundred bases. Resection at the radiation-induced DSBs continues so that by the time of significant repair of DSBs at 1 hr there is about 1-2 kb resection per DSB end. The PFGE-shift is comparable in WT and recombination-defective rad52 and rad51 strains but somewhat delayed in exo1 mutants. However, in rad50 and mre11 null mutants the initiation and generation of resected ends at radiation-induced DSB ends is greatly reduced in G2/M. Thus, the Rad50/Mre11/Xrs2 complex is responsible for rapid processing of most damaged ends into substrates that subsequently undergo recombinational repair. A similar requirement was found for RAD50 in asynchronously growing cells. Among the few molecules exhibiting shift in the rad50 mutant, the residual resection is consistent with resection at only one of the DSB ends. Surprisingly, within 1 hr after irradiation, double-length linear molecules are detected in the WT and rad50, but not in rad52, strains that are likely due to crossovers that are largely resection- and RAD50-independent.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. - PubMed
-
- Lieber MR. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. - PubMed
-
- Nickoloff JA, Singer JD, Hoekstra MF, Heffron F. Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol. 1989;207:527–541. - PubMed
-
- Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst) 2006;5:1021–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

