Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats
- PMID: 19763257
- PMCID: PMC2739291
- DOI: 10.1371/journal.pone.0007065
Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats
Erratum in
- PLoS One. 2013;8(5). doi:10.1371/annotation/80ebddbb-4dda-4785-a5f2-ffd65ec1c79b. Tironi-Farinatti, Carla [corrected to Tironi-Farinati, Carla]
Abstract
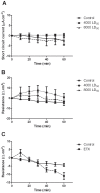
Epsilon toxin is a potent neurotoxin produced by Clostridium perfringens types B and D, an anaerobic bacterium that causes enterotoxaemia in ruminants. In the affected animal, it causes oedema of the lungs and brain by damaging the endothelial cells, inducing physiological and morphological changes. Although it is believed to compromise the intestinal barrier, thus entering the gut vasculature, little is known about the mechanism underlying this process. This study characterizes the effects of epsilon toxin on fluid transport and bioelectrical parameters in the small intestine of mice and rats. The enteropooling and the intestinal loop tests, together with the single-pass perfusion assay and in vitro and ex vivo analysis in Ussing's chamber, were all used in combination with histological and ultrastructural analysis of mice and rat small intestine, challenged with or without C. perfringens epsilon toxin. Luminal epsilon toxin induced a time and concentration dependent intestinal fluid accumulation and fall of the transepithelial resistance. Although no evident histological changes were observed, opening of the mucosa tight junction in combination with apoptotic changes in the lamina propria were seen with transmission electron microscopy. These results indicate that C. perfringens epsilon toxin alters the intestinal permeability, predominantly by opening the mucosa tight junction, increasing its permeability to macromolecules, and inducing further degenerative changes in the lamina propria of the bowel.
Conflict of interest statement
Figures





Similar articles
-
Clostridium perfringens type-D enterotoxaemia in cattle: the diagnostic significance of intestinal epsilon toxin.Vet Rec. 2015 Oct 17;177(15):390. doi: 10.1136/vr.103097. Epub 2015 Oct 1. Vet Rec. 2015. PMID: 26428898
-
Effects of Clostridium perfringens iota toxin in the small intestine of mice.Anaerobe. 2017 Dec;48:83-88. doi: 10.1016/j.anaerobe.2017.07.007. Epub 2017 Jul 29. Anaerobe. 2017. PMID: 28764997
-
The early effects of Clostridium perfringens type D epsilon toxin in ligated intestinal loops of goats and sheep.Vet Res Commun. 2003 Apr;27(3):231-41. doi: 10.1023/a:1023348708599. Vet Res Commun. 2003. PMID: 12777097
-
New insights into Clostridium perfringens epsilon toxin activation and action on the brain during enterotoxemia.Anaerobe. 2016 Oct;41:27-31. doi: 10.1016/j.anaerobe.2016.06.006. Epub 2016 Jun 16. Anaerobe. 2016. PMID: 27321761 Review.
-
Clostridium perfringens epsilon toxin: the third most potent bacterial toxin known.Anaerobe. 2014 Dec;30:102-7. doi: 10.1016/j.anaerobe.2014.08.016. Epub 2014 Sep 16. Anaerobe. 2014. PMID: 25234332 Review.
Cited by
-
The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of Clostridium perfringens ε-Toxin.PLoS Pathog. 2015 May 20;11(5):e1004896. doi: 10.1371/journal.ppat.1004896. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25993478 Free PMC article.
-
Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens ε-toxin.PLoS One. 2011 Mar 11;6(3):e17787. doi: 10.1371/journal.pone.0017787. PLoS One. 2011. PMID: 21412435 Free PMC article.
-
Neck Muscle Hemorrhage in an Alpine Kid Following Enterotoxemia: a New Necropsy Finding.Arch Razi Inst. 2024 Dec 31;79(6):1381-1386. doi: 10.32592/ARI.2024.79.6.1381. eCollection 2024 Dec. Arch Razi Inst. 2024. PMID: 40599437 Free PMC article.
-
Does Clostridium Perfringens Epsilon Toxin Mimic an Auto-Antigen Involved in Multiple Sclerosis?Toxins (Basel). 2025 Jan 7;17(1):27. doi: 10.3390/toxins17010027. Toxins (Basel). 2025. PMID: 39852980 Free PMC article.
-
Toxin plasmids of Clostridium perfringens.Microbiol Mol Biol Rev. 2013 Jun;77(2):208-33. doi: 10.1128/MMBR.00062-12. Microbiol Mol Biol Rev. 2013. PMID: 23699255 Free PMC article. Review.
References
-
- McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins T. The enterotoxic clostridia, In: Dworkin M, Falkow S, Rosenburg E, Schleifer KH, Stackebrandt E, editors. The prokaryotes, vol. 4. New York, NY: Springer-Verlag; 2006. pp. 698–752.
-
- Minami J, Katayama S, Matsushita O, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41:527–535. - PubMed
-
- Uzal FA. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Anaerobe. 2004;10:135–143. - PubMed
-
- Finnie JW. Pathogenesis of brain damage produced in sheep by Clostridium perfringens type D epsilon toxin: a review. Aust Vet J. 2003;81:219–221. - PubMed
-
- Uzal FA, Kelly WR, Morris WE, Bermudez J, Baisón M. The pathology of peracute experimental Clostridium perfringens type D enterotoxaemia in sheep. J Vet Diagn Invest. 2004;16:403–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

