Transcriptional signature and memory retention of human-induced pluripotent stem cells
- PMID: 19763270
- PMCID: PMC2741600
- DOI: 10.1371/journal.pone.0007076
Transcriptional signature and memory retention of human-induced pluripotent stem cells
Abstract
Genetic reprogramming of somatic cells to a pluripotent state (induced pluripotent stem cells or iPSCs) by over-expression of specific genes has been accomplished using mouse and human cells. However, it is still unclear how similar human iPSCs are to human Embryonic Stem Cells (hESCs). Here, we describe the transcriptional profile of human iPSCs generated without viral vectors or genomic insertions, revealing that these cells are in general similar to hESCs but with significant differences. For the generation of human iPSCs without viral vectors or genomic insertions, pluripotent factors Oct4 and Nanog were cloned in episomal vectors and transfected into human fetal neural progenitor cells. The transient expression of these two factors, or from Oct4 alone, resulted in efficient generation of human iPSCs. The reprogramming strategy described here revealed a potential transcriptional signature for human iPSCs yet retaining the gene expression of donor cells in human reprogrammed cells free of viral and transgene interference. Moreover, the episomal reprogramming strategy represents a safe way to generate human iPSCs for clinical purposes and basic research.
Conflict of interest statement
Figures

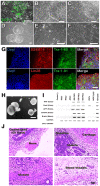
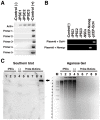


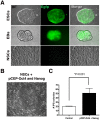

References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3:1180–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

