Capecitabine treatment patterns in patients with gastroesophageal cancer in the United States
- PMID: 19764093
- PMCID: PMC2747062
- DOI: 10.3748/wjg.15.4415
Capecitabine treatment patterns in patients with gastroesophageal cancer in the United States
Abstract
Aim: To assess the use of capecitabine-based therapy and associated complication rates in patients with gastroesophageal cancer (GEC) in a real-world treatment setting.
Methods: Patients with claims between 2004 and 2005 were identified from the Thomson Reuters MarketScan databases. Capecitabine regimens were compared with 5-fluorouracil (5-FU) and other chemotherapy regimens, and were stratified by treatment setting.
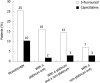
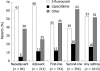
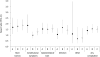
Results: We identified 1013 patients with GEC: approximately half had treatment initiated with a 5-FU regimen, whereas 11% had therapy initiated with a capecitabine regimen. The mean capecitabine dose overall was 2382 +/- 1118 mg/d, and capecitabine was used as monotherapy more often than in combination. Overall, 5-FU regimens were the most common treatment option in neoadjuvant and adjuvant settings, while other non-capecitabine regimens were used more widely in first- and second-line settings. The overall unadjusted complication rate for capecitabine regimens was about half of that seen with 5-FU regimens. In multivariate analyses, capecitabine recipients had a 51% (95% CI: 26%-81%) lower risk of developing any complication than 5-FU recipients did. The risk of developing bone marrow, constitutional, gastrointestinal tract, infectious, or skin complications was lower with capecitabine therapy than with 5-FU.
Conclusion: Capecitabine appeared to have a favorable side effect profile compared with 5-FU, which indicates that it may be a treatment option for GEC.
Figures
References
-
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. - PubMed
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Esophageal cancer. Version 2. Fort Washington, PA: National Comprehensive Cancer Network. 2007. Available from: URL: http://www.nccn.org.
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Gastric cancer. Version 2. Fort Washington, PA: National Comprehensive Cancer Network. 2007. Available from: URL: http://www.nccn.org.
-
- American Cancer Society. Cancer facts and figures 2006. Atlanta, GA: American Cancer Society; 2006. [Accessed September 5, 2008]. Available from: http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf.
-
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

