Assembly with the Na,K-ATPase alpha(1) subunit is required for export of beta(1) and beta(2) subunits from the endoplasmic reticulum
- PMID: 19764716
- PMCID: PMC2987690
- DOI: 10.1021/bi901438z
Assembly with the Na,K-ATPase alpha(1) subunit is required for export of beta(1) and beta(2) subunits from the endoplasmic reticulum
Abstract
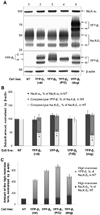
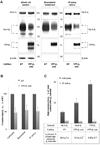
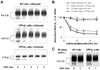
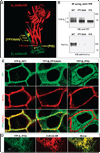
The level of the heterodimeric Na,K-ATPase is tightly controlled in epithelia to maintain appropriate transport function. The catalytic Na,K-ATPase alpha subunit is not able to exit the ER or catalyze ion transport unless assembled with the beta subunit. However, requirements for the ER exit of the Na,K-ATPase beta subunit that plays an additional, ion-transport-independent, role in intercellular adhesion are not clear. Exogenous beta(1) or beta(2) subunits expressed in renal MDCK cells replace endogenous beta(1) subunits in the alpha-beta complexes in the ER, resulting in a decrease in the amount of the alpha(1)-bound endogenous beta(1) subunits by 47-61% with no change in the amount of alpha(1) subunits. Disruption of the alpha(1)-beta association by mutations in defined alpha(1)-interacting regions of either beta(1) or beta(2) subunits results in the ER retention and rapid degradation of unassembled mutants. Hence, the ER quality control system allows export only of assembled alpha-beta complexes to the Golgi, thereby maintaining an equimolar ratio of alpha and beta subunits in the plasma membrane, whereas the number of alpha(1) subunits in the ER determines the amount of the alpha-beta complexes.
Figures

References
-
- Xie Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann. N.Y. Acad. Sci. 2003;986:497–503. - PubMed
-
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 1998;275:F633–F650. - PubMed
-
- Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. - PubMed
-
- Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

