QUARITE (quality of care, risk management and technology in obstetrics): a cluster-randomized trial of a multifaceted intervention to improve emergency obstetric care in Senegal and Mali
- PMID: 19765280
- PMCID: PMC2758868
- DOI: 10.1186/1745-6215-10-85
QUARITE (quality of care, risk management and technology in obstetrics): a cluster-randomized trial of a multifaceted intervention to improve emergency obstetric care in Senegal and Mali
Abstract
Background: Maternal and perinatal mortality are major problems for which progress in sub-Saharan Africa has been inadequate, even though childbirth services are available, even in the poorest countries. Reducing them is the aim of two of the main Millennium Development Goals. Many initiatives have been undertaken to remedy this situation, such as the Advances in Labour and Risk Management (ALARM) International Program, whose purpose is to improve the quality of obstetric services in low-income countries. However, few interventions have been evaluated, in this context, using rigorous methods for analyzing effectiveness in terms of health outcomes. The objective of this trial is to evaluate the effectiveness of the ALARM International Program (AIP) in reducing maternal mortality in referral hospitals in Senegal and Mali. Secondary goals include evaluation of the relationships between effectiveness and resource availability, service organization, medical practices, and satisfaction among health personnel.
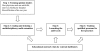
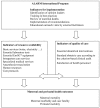
Methods/design: This is an international, multi-centre, controlled cluster-randomized trial of a complex intervention. The intervention is based on the concept of evidence-based practice and on a combination of two approaches aimed at improving the performance of health personnel: 1) Educational outreach visits; and 2) the implementation of facility-based maternal death reviews. The unit of intervention is the public health facility equipped with a functional operating room. On the basis of consent provided by hospital authorities, 46 centres out of 49 eligible were selected in Mali and Senegal. Using randomization stratified by country and by level of care, 23 centres will be allocated to the intervention group and 23 to the control group. The intervention will last two years. It will be preceded by a pre-intervention one-year period for baseline data collection. A continuous clinical data collection system has been set up in all participating centres. This, along with the inventory of resources and the satisfaction surveys administered to the health personnel, will allow us to measure results before, during, and after the intervention. The overall rate of maternal mortality measured in hospitals during the post-intervention period (Year 4) is the primary outcome. The evaluation will also include cost-effectiveness.
Figures



Similar articles
-
Quality of care, risk management, and technology in obstetrics to reduce hospital-based maternal mortality in Senegal and Mali (QUARITE): a cluster-randomised trial.Lancet. 2013 Jul 13;382(9887):146-57. doi: 10.1016/S0140-6736(13)60593-0. Epub 2013 May 28. Lancet. 2013. PMID: 23721752 Clinical Trial.
-
Effect of maternal death reviews and training on maternal mortality among cesarean delivery: post-hoc analysis of a cluster-randomized controlled trial.Eur J Obstet Gynecol Reprod Biol. 2015 Feb;185:174-80. doi: 10.1016/j.ejogrb.2014.12.023. Epub 2015 Jan 3. Eur J Obstet Gynecol Reprod Biol. 2015. PMID: 25590501 Clinical Trial.
-
Effect of a facility-based multifaceted intervention on the quality of obstetrical care: a cluster randomized controlled trial in Mali and Senegal.BMC Pregnancy Childbirth. 2013 Jan 25;13:24. doi: 10.1186/1471-2393-13-24. BMC Pregnancy Childbirth. 2013. PMID: 23351269 Free PMC article. Clinical Trial.
-
Care prior to and during subsequent pregnancies following stillbirth for improving outcomes.Cochrane Database Syst Rev. 2018 Dec 17;12(12):CD012203. doi: 10.1002/14651858.CD012203.pub2. Cochrane Database Syst Rev. 2018. PMID: 30556599 Free PMC article.
-
Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth.Cochrane Database Syst Rev. 2020 May 26;5(5):CD013633. doi: 10.1002/14651858.CD013633. Cochrane Database Syst Rev. 2020. PMID: 32452555 Free PMC article.
Cited by
-
Care during labor and birth for the prevention of intrapartum-related neonatal deaths: a systematic review and Delphi estimation of mortality effect.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S10. doi: 10.1186/1471-2458-11-S3-S10. BMC Public Health. 2011. PMID: 21501427 Free PMC article.
-
Predicting in-hospital maternal mortality in Senegal and Mali.PLoS One. 2013 May 30;8(5):e64157. doi: 10.1371/journal.pone.0064157. Print 2013. PLoS One. 2013. PMID: 23737972 Free PMC article.
-
What the percentage of births in facilities does not measure: readiness for emergency obstetric care and referral in Senegal.BMJ Glob Health. 2020 Mar 3;5(3):e001915. doi: 10.1136/bmjgh-2019-001915. eCollection 2020. BMJ Glob Health. 2020. PMID: 32201621 Free PMC article.
-
Predictors of uterine rupture in a large sample of women in Senegal and Mali: cross-sectional analysis of QUARITE trial data.BMC Pregnancy Childbirth. 2018 Nov 1;18(1):432. doi: 10.1186/s12884-018-2064-y. BMC Pregnancy Childbirth. 2018. PMID: 30382820 Free PMC article. Clinical Trial.
-
Application effect of continuous quality improvement measures on patient satisfaction and quality of life in gynecological nursing.Am J Transl Res. 2021 Jun 15;13(6):6391-6398. eCollection 2021. Am J Transl Res. 2021. PMID: 34306378 Free PMC article.
References
-
- AMDD Working Group on Indicators Program note: using UN process indicators to assess needs in emergency obstetric services: Benin and Chad. Int J Gynaecol Obstet. 2004;86:110–20. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

