The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse
- PMID: 19765557
- PMCID: PMC3274960
- DOI: 10.1016/j.brainres.2009.09.036
The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse
Abstract
The interaction between the stress axis and endogenous opioid systems has gained substantial clinical attention as it is increasingly recognized that stress predisposes to opiate abuse. For example, stress has been implicated as a risk factor in vulnerability to the initiation and maintenance of opiate abuse and is thought to play an important role in relapse in subjects with a history of abuse. Numerous reports indicating that stress alters individual sensitivity to opiates suggest that prior stress can influence the pharmacodynamics of opiates that are used in clinical settings. Conversely, the effects of opiates on different components of the stress axis can impact on individual responsivity to stressors and potentially predispose individuals to stress-related psychiatric disorders. One site at which opiates and stress substrates may interact to have global effects on behavior is within the locus coeruleus (LC), the major brain norepinephrine (NE)-containing nucleus. This review summarizes our current knowledge regarding the anatomical and neurochemical afferent regulation of the LC. It then presents physiological studies demonstrating opposing interactions between opioids and stress-related neuropeptides in the LC and summarizes results showing that chronic morphine exposure sensitizes the LC-NE system to corticotropin releasing factor and stress. Finally, new evidence for novel presynaptic actions of kappa-opioids on LC afferents is provided that adds another dimension to our model of how this central NE system is co-regulated by opioids and stress-related peptides.
Copyright 2009. Published by Elsevier B.V.
Figures

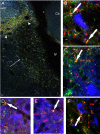
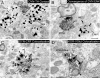
Similar articles
-
Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses.Adv Pharmacol. 2013;68:405-20. doi: 10.1016/B978-0-12-411512-5.00019-1. Adv Pharmacol. 2013. PMID: 24054155 Free PMC article. Review.
-
Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity.Psychopharmacology (Berl). 2001 Dec;158(4):331-42. doi: 10.1007/s002130000673. Epub 2001 Mar 14. Psychopharmacology (Berl). 2001. PMID: 11797054 Review.
-
Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence.Neuropsychopharmacology. 2013 Sep;38(10):1833-43. doi: 10.1038/npp.2013.117. Epub 2013 May 10. Neuropsychopharmacology. 2013. PMID: 23660707 Free PMC article.
-
Sex differences in morphine-induced trafficking of mu-opioid and corticotropin-releasing factor receptors in locus coeruleus neurons.Brain Res. 2019 Mar 1;1706:75-85. doi: 10.1016/j.brainres.2018.11.001. Epub 2018 Nov 2. Brain Res. 2019. PMID: 30391476
-
Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal.Neuroscience. 2013 Sep 17;248:637-54. doi: 10.1016/j.neuroscience.2013.04.034. Epub 2013 Apr 24. Neuroscience. 2013. PMID: 23624062 Free PMC article. Review.
Cited by
-
Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses.Adv Pharmacol. 2013;68:405-20. doi: 10.1016/B978-0-12-411512-5.00019-1. Adv Pharmacol. 2013. PMID: 24054155 Free PMC article. Review.
-
Differential effects of inescapable stress on locus coeruleus GRK3, alpha2-adrenoceptor and CRF1 receptor levels in learned helpless and non-helpless rats: a potential link to stress resilience.Behav Brain Res. 2011 Aug 1;221(1):25-33. doi: 10.1016/j.bbr.2011.02.018. Epub 2011 Feb 17. Behav Brain Res. 2011. PMID: 21333691 Free PMC article.
-
Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression.Mol Psychiatry. 2013 Feb;18(2):166-73. doi: 10.1038/mp.2012.24. Epub 2012 Apr 17. Mol Psychiatry. 2013. PMID: 22508464 Free PMC article.
-
Differential Susceptibility of the Developing Brain to Contextual Adversity and Stress.Neuropsychopharmacology. 2016 Jan;41(1):142-62. doi: 10.1038/npp.2015.294. Epub 2015 Sep 22. Neuropsychopharmacology. 2016. PMID: 26391599 Free PMC article. Review.
-
Pannexin-1 channel inhibition alleviates opioid withdrawal in rodents by modulating locus coeruleus to spinal cord circuitry.Nat Commun. 2024 Jul 24;15(1):6264. doi: 10.1038/s41467-024-50657-7. Nat Commun. 2024. PMID: 39048565 Free PMC article.
References
-
- Ackley MA, Hurley RW, Virnich DE, Hammond DL. A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain. 2001;91:377–388. - PubMed
-
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. - PubMed
-
- Aghajanian GK, Wang YY. Common alpha 2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology. 1987;26:793–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

