Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner
- PMID: 19765656
- PMCID: PMC6817335
- DOI: 10.1016/j.matbio.2009.09.003
Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner
Abstract
Previously, we described a profound impact of structural conformation of collagen matrix on osteogenic and adipogenic differentiation of bone marrow stromal cells. Thus, a marginal p38-independent adipogenesis on native collagen I matrix contrasts with an efficient p38-dependent differentiation on denatured collagen I. An efficient Hsp90-dependent osteogenesis occurs on native collagen I matrix but not on its denatured counterpart where it is insignificant and proceeds in an Hsp90-independent manner. Whereas only marginal osteogenesis and no detectable adipogenesis of bone marrow stromal cells occur on native collagen IV, the same matrix supports a highly efficient adipogenesis in denatured structural state. The present study addresses the opposite direction in the flow of cell-matrix interaction, namely the cells' influence on structural state of collagen matrix, and tests the possibility that differentiating bone marrow stromal cells may adjust the expression phenotype of MMP and TIMP in such a way that, if translated into matrix modification, would facilitate the maintenance of collagen matrix in or its modification into structural state optimal for the ongoing differentiation process. The results obtained indicate that this is indeed the case. In bone marrow stromal cells stimulated to undergo adipogenesis the expression of MMP increases and that of TIMP decreases. In cells induced to undergo osteogenesis the opposite is true: MMP/TIMP expression is adjusted in a manner that, if translated into matrix modification, could promote the native structural conformation optimal for this type of differentiation. The results obtained also indicate that the observed adjustment in MMP/TIMP expression phenotype might be an early differentiation event and that differentiation stimulation alone might be sufficient to trigger it even on matrices not favorable to a given type of differentiation. The findings of the present study raise significant questions and indicate directions for further experimentation.
Figures


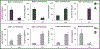
References
-
- Altman G, Horan R, Martin I, Farhadi J 2002. Cell differentiation by mechanical stress. FASEB J 16, 270–2. - PubMed
-
- Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, and Engler JA 1993. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med 4, 197–250. - PubMed
-
- Borden P, Heller R. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. 1997. Crit Rev Eukaryotic Gene Expr 7, 159–178 - PubMed
-
- Brinckerhoff CE, Matrisian LM. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell. Biol 3, 207–214. - PubMed
-
- Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E and Tartare-Deckert S 2003. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J. Biol. Chem 278, 11888–11896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

