Restoring barrier function to acid damaged bladder by intravesical chondroitin sulfate
- PMID: 19765766
- PMCID: PMC3157297
- DOI: 10.1016/j.juro.2009.07.013
Restoring barrier function to acid damaged bladder by intravesical chondroitin sulfate
Abstract
Purpose: Chondroitin sulfate (Stellar Pharmaceuticals, London, Ontario, Canada), which is less expensive and more inert than heparinoids, hyaluronan or pentosan polysulfate, has been introduced to restore the barrier function lost due to epithelial dysfunction in interstitial cystitis cases. To our knowledge chondroitin sulfate binding to damaged bladder as a function of the urinary pH range, its efficacy in restoring the bladder permeability barrier and the capacity of the damaged bladder to bind chondroitin sulfate have not been determined previously.
Materials and methods: Chondroitin sulfate binding to bladder urothelium was investigated quantitatively using chondroitin sulfate highly labeled with Texas Red(R) and quantitative fluorescence microscopy in a mouse model of urothelial acid damage. The efficacy of restoring barrier function was determined using the passage of intravesically instilled (86)Rb, a potassium ion mimetic, through the urothelium into the bloodstream in a rat model of bladder damage. The binding capacity of acid damaged bladder was determined by fluorometry.
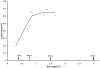
Results: Chondroitin sulfate bound tightly and exclusively to the mouse bladder surface damaged by acid but showed only minimal binding to undamaged bladder. There was no systematic variation in pH. The model showed some variability in the degree of damage induced. In rats chondroitin sulfate instillation restored permeability to (86)Rb to control levels. Binding was saturable at a mean +/- SEM 0.67 +/- 0.13 mg/cm(2) of the bladder surface.
Conclusions: Chondroitin sulfate binds preferentially to damaged urothelium and restores the impermeability barrier. This suggests that the glycosaminoglycan layer is a major contributor to bladder urothelial impermeability. As determined by binding capacity, the dose applied in humans in Canada (400 mg per instillation) is sufficient to achieve maximum efficacy.
Figures






Comment in
-
The urothelial glycosaminoglycan layer revisited.J Urol. 2009 Nov;182(5):2103-4. doi: 10.1016/j.juro.2009.08.080. Epub 2009 Sep 16. J Urol. 2009. PMID: 19758629 No abstract available.
References
-
- Hanno P, Nordling J, van OA. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 2008;18:353. - PubMed
-
- Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal Expression of Molecular Markers for Bladder Impermeability and Differentiation in Urothelium of Interstitial Cystitis Patients. J Urol. 2004;171:1554. - PubMed
-
- Chai TC, Keay S. New theories in interstitial cystitis. Nat Clin Pract Urol. 2004;1:85. - PubMed
-
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

