Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening
- PMID: 19765988
- PMCID: PMC2755619
- DOI: 10.1016/j.cub.2009.07.069
Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening
Abstract
Background: A number of adhesion-mediated signaling pathways and cell-cycle events have been identified that regulate cell proliferation, yet studies to date have been unable to determine which of these pathways control mitogenesis in response to physiologically relevant changes in tissue elasticity. In this report, we use hydrogel-based substrata matched to biological tissue stiffness to investigate the effects of matrix elasticity on the cell cycle.
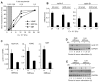
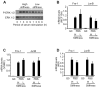
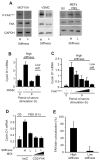
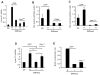
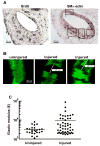
Results: We find that physiological tissue stiffness acts as a cell-cycle inhibitor in mammary epithelial cells and vascular smooth muscle cells; subcellular analysis in these cells, mouse embryonic fibroblasts, and osteoblasts shows that cell-cycle control by matrix stiffness is widely conserved. Remarkably, most mitogenic events previously documented as extracellular matrix (ECM)/integrin-dependent proceed normally when matrix stiffness is altered in the range that controls mitogenesis. These include ERK activity, immediate-early gene expression, and cdk inhibitor expression. In contrast, FAK-dependent Rac activation, Rac-dependent cyclin D1 gene induction, and cyclin D1-dependent Rb phosphorylation are strongly inhibited at physiological tissue stiffness and rescued when the matrix is stiffened in vitro. Importantly, the combined use of atomic force microscopy and fluorescence imaging in mice shows that comparable increases in tissue stiffness occur at sites of cell proliferation in vivo.
Conclusions: Matrix remodeling associated with pathogenesis is in itself a positive regulator of the cell cycle through a highly selective effect on integrin-dependent signaling to FAK, Rac, and cyclin D1.
Figures

References
-
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. - PubMed
-
- Fringer J, Grinnell F. Fibroblast quiescence in floating or released collagen matrices: contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem. 2001;276:31047–31052. - PubMed
-
- Rosenfeldt H, Grinnell F. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem. 2000;275:3088–3092. - PubMed
-
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. - PubMed
-
- Wall SJ, Zhong ZD, DeClerck YA. The cyclin-dependent kinase inhibitors p15INK4B and p21CIP1 are critical regulators of fibrillar collagen-induced tumor cell cycle arrest. J Biol Chem. 2007;282:24471–24476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

