Innate cells and T helper 2 cell immunity in airway inflammation
- PMID: 19766085
- PMCID: PMC3353417
- DOI: 10.1016/j.immuni.2009.08.014
Innate cells and T helper 2 cell immunity in airway inflammation
Abstract
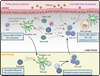
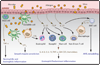
Activated mast cells, eosinophils, and basophils infiltrate the airways of asthmatics as a result of an overexuberant T helper 2 (Th2) cell immune response that drives the production of IgE, primes mast cells and basophils, and promotes tissue eosinophilia and mast cell hyperplasia. Recent evidence demonstrates that these innate effectors can be activated outside of this classical Th2 cell paradigm and that they have additional roles in promoting the development of innate and adaptive pulmonary inflammation. There is also an appreciation for the role of airway epithelial cells in orchestrating allergic pulmonary inflammation. Emerging data from basic research highlight the involvement of many unique pathways in the inflammation triggered by complex native allergens and microbes at the airway mucosal surface. Here, we review the role of effector cells and airway epithelial cells in augmenting and, at times, bypassing traditional Th2 cell-mediated allergic inflammation.
Figures
References
-
- Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. - PubMed
-
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. - PubMed
-
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon B-RP, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007a;204:253–258. - PMC - PubMed

