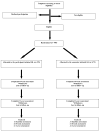
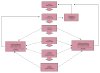
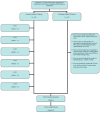
Trial design: The St. Jude Children's Research Hospital Cancer Survivors Tobacco Quit Line study
- PMID: 19766734
- PMCID: PMC2818168
- DOI: 10.1016/j.cct.2009.09.004
Trial design: The St. Jude Children's Research Hospital Cancer Survivors Tobacco Quit Line study
Abstract
Nearly, one-fifth of childhood cancer survivors (CCSs) smoke cigarettes. Because CCSs are already at greater medical smoking-related risks, targeting them for smoking cessation efforts is a high priority. One of the major challenges with smoking cessation in CCSs is how to reach such a geographically dispersed population. This study aims to demonstrate that these challenges can be overcome through the use of telephone-based tobacco quit lines (QLs). This report describes the design of the St. Jude Cancer Survivor Tobacco QL study, which is a randomized controlled clinical trial that will examine the long-term (1-year) efficacy of a counselor initiated vs. participant initiated tobacco QL with adjunctive nicotine replacement therapy (NRT) in both groups. Participants (N=950) will be recruited nationally and randomly assigned to one of the two interventions. The counselor initiated intervention includes six scheduled telephone sessions of a behavioral intervention and provision of 8 weeks of NRT. The participant initiated intervention allows the participant to call the QL at their convenience, but includes the same six telephone sessions and provision of 2 weeks of NRT. Both groups will receive two follow-up phone calls at 8 weeks and 1 year after enrollment to assess their smoking status. The primary outcome measure is cotinine-validated self-reported smoking abstinence at 1-year follow-up. Results from this study will provide the first evidence about the efficacy of intensive QL cessation intervention in this high-risk population. Such evidence can lead as well to the dissemination of this intervention to other medically compromised populations.
Published by Elsevier Inc.
Figures
Comment in
-
Randomization, permuted blocks, masking, allocation concealment, and selection bias in the Tobacco Quit Line Study.Contemp Clin Trials. 2010 May;31(3):201. doi: 10.1016/j.cct.2010.02.004. Epub 2010 Feb 25. Contemp Clin Trials. 2010. PMID: 20219696 No abstract available.
References
-
- Hewitt M, Weiner SL, Simone JV. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Cancer Policy Board; 2003. - PubMed
-
- Reis LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics review, 1973–1999. National Cancer Institute; Bethesda, MD: 2002. http://seer.cancer.gov/csr/1973_1999/
-
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. http://seer.cancer.gov/csr/1975_2004/
-
- Emmons KM, Butterfield RM, Puleo E, Park ER, Mertens A, Gritz ER, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health study. J Clin Oncol. 2003;21:189–196. - PubMed
-
- CDC. Cigarette Smoking Among Adults - United States, 2006. MMWR. 2007;56:1157–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous