Chapter 5. Nuclear actin-related proteins in epigenetic control
- PMID: 19766970
- PMCID: PMC2800988
- DOI: 10.1016/S1937-6448(09)77005-4
Chapter 5. Nuclear actin-related proteins in epigenetic control
Abstract
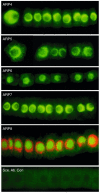
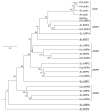
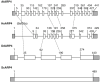
The nuclear actin-related proteins (ARPs) share overall structure and low-level sequence homology with conventional actin. They are indispensable subunits of macromolecular machines that control chromatin remodeling and modification leading to dynamic changes in DNA structure, transcription, and DNA repair. Cellular, genetic, and biochemical studies suggest that the nuclear ARPs are essential to the epigenetic control of the cell cycle and cell proliferation in all eukaryotes, while in plants and animals they also exert epigenetic controls over most stages of multicellular development including organ initiation, the switch to reproductive development, and senescence and programmed cell death. A theme emerging from plants and animals is that in addition to their role in controlling the general compaction of DNA and gene silencing, isoforms of nuclear ARP-containing chromatin complexes have evolved to exert dynamic epigenetic control over gene expression and different phases of multicellular development. Herein, we explore this theme by examining nuclear ARP phylogeny, activities of ARP-containing chromatin remodeling complexes that lead to epigenetic control, expanding developmental roles assigned to several animal and plant ARP-containing complexes, the evidence that thousands of ARP complex isoforms may have evolved in concert with multicellular development, and ARPs in human disease.
Figures


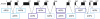



Similar articles
-
Nuclear actin-related proteins at the core of epigenetic control.Plant Signal Behav. 2010 May;5(5):518-22. doi: 10.4161/psb.10986. Epub 2010 May 1. Plant Signal Behav. 2010. PMID: 21228632 Free PMC article.
-
Actin-related proteins in chromatin-level control of the cell cycle and developmental transitions.Trends Cell Biol. 2007 Jul;17(7):325-32. doi: 10.1016/j.tcb.2007.06.001. Epub 2007 Jul 20. Trends Cell Biol. 2007. PMID: 17643304 Review.
-
Actin-related proteins (Arps): conformational switches for chromatin-remodeling machines?Bioessays. 2000 Jul;22(7):666-72. doi: 10.1002/1521-1878(200007)22:7<666::AID-BIES9>3.0.CO;2-Y. Bioessays. 2000. PMID: 10878579 Review.
-
Multiple actin-related proteins of Saccharomyces cerevisiae are present in the nucleus.J Biochem. 2000 Oct;128(4):665-71. doi: 10.1093/oxfordjournals.jbchem.a022799. J Biochem. 2000. PMID: 11011149
-
Nuclear actin and actin-related proteins in chromatin dynamics.Curr Opin Cell Biol. 2007 Jun;19(3):326-30. doi: 10.1016/j.ceb.2007.04.009. Epub 2007 Apr 27. Curr Opin Cell Biol. 2007. PMID: 17467255 Review.
Cited by
-
Nuclear actin-related protein is required for chromosome segregation in Toxoplasma gondii.Mol Biochem Parasitol. 2012 Jan;181(1):7-16. doi: 10.1016/j.molbiopara.2011.09.006. Epub 2011 Sep 22. Mol Biochem Parasitol. 2012. PMID: 21963440 Free PMC article.
-
Loss of Arp1, a putative actin-related protein, triggers filamentous and invasive growth and impairs pathogenicity in Candida albicans.Comput Struct Biotechnol J. 2020 Dec 1;18:4002-4015. doi: 10.1016/j.csbj.2020.11.034. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33363697 Free PMC article.
-
The evolution of epitype.Plant Cell. 2010 Jun;22(6):1658-66. doi: 10.1105/tpc.110.075481. Epub 2010 Jun 15. Plant Cell. 2010. PMID: 20551346 Free PMC article.
-
The Nature of Actin-Family Proteins in Chromatin-Modifying Complexes.Front Genet. 2018 Sep 25;9:398. doi: 10.3389/fgene.2018.00398. eCollection 2018. Front Genet. 2018. PMID: 30319687 Free PMC article. Review.
-
ACTIN-RELATED PROTEIN6 Regulates Female Meiosis by Modulating Meiotic Gene Expression in Arabidopsis.Plant Cell. 2014 Apr;26(4):1612-1628. doi: 10.1105/tpc.113.120576. Epub 2014 Apr 15. Plant Cell. 2014. PMID: 24737671 Free PMC article.
References
-
- Adam RD. The Giardia lamblia genome. Int. J. Parasitol. 2000;30:475–484. - PubMed
-
- Adams PD. Remodeling chromatin for senescence. Aging Cell. 2007a;6:425–427. - PubMed
-
- Adl SM, Leander BS, Simpson AG, Archibald JM, Anderson OR, Bass D, et al. Diversity, nomenclature, and taxonomy of protists. Syst. Biol. 2007;56:684–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

