Complex phosphatase regulation of Ca2+-activated Cl- currents in pulmonary arterial smooth muscle cells
- PMID: 19767392
- PMCID: PMC2781665
- DOI: 10.1074/jbc.M109.050401
Complex phosphatase regulation of Ca2+-activated Cl- currents in pulmonary arterial smooth muscle cells
Abstract
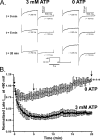
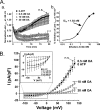
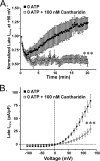
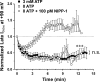
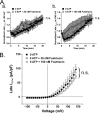
The present study was undertaken to determine whether the two ubiquitously expressed Ca(2+)-independent phosphatases PP1 and PP2A regulate Ca(2+)-activated Cl(-) currents (I(Cl(Ca))) elicited by 500 nM [Ca(2+)](i) in rabbit pulmonary artery (PA) myocytes dialyzed with or without 3 mM ATP. Reverse transcription-PCR experiments revealed the expression of PP1alpha, PP1beta/delta, PP1gamma, PP2Aalpha, PP2Abeta, PP2Balpha (calcineurin (CaN) Aalpha), and PP2Bbeta (CaN Abeta) but not PP2Bgamma (CaN Agamma) in rabbit PA. Western blot and immunofluorescence experiments confirmed the presence of all three PP1 isoforms and PP2A. Intracellular dialysis with a peptide inhibitor of calcineurin (CaN-AIP); the non-selective PP1/PP2A inhibitors okadaic acid (0.5, 10, or 30 nM), calyculin A (10 nM), or cantharidin (100 nM); and the selective PP1 inhibitor NIPP-1 (100 pM) potently antagonized the recovery of I(Cl(Ca)) in cells dialyzed with no ATP, whereas the PP2A-selective antagonist fostriecin (30 or 150 nM) was ineffective. The combined application of okadaic acid (10 nM) and CaN-autoinhibitory peptide (50 microM) did not potentiate the response of I(Cl(Ca)) in 0 ATP produced by maximally inhibiting CaN or PP1/PP2A alone. Consistent with the non-additive effects of either classes of phosphatases, the PP1 inhibitor NIPP-1 (100 pM) antagonized the recovery of I(Cl(Ca)) induced by exogenous CaN Aalpha (0.5 microM). These results demonstrate that I(Cl(Ca)) in PA myocytes is regulated by CaN and PP1 and/or PP2A. Our data also suggest the existence of a functional link between these two classes of phosphatases.
Figures




References
-
- Chipperfield A. R., Harper A. A. (2000) Prog. Biophys. Mol. Biol. 74, 175–221 - PubMed
-
- Large W. A., Wang Q. (1996) Am. J. Physiol. 271, C435–C454 - PubMed
-
- Leblanc N., Ledoux J., Saleh S., Sanguinetti A., Angermann J., O'Driscoll K., Britton F., Perrino B. A., Greenwood I. A. (2005) Can J. Physiol. Pharmacol. 83, 541–556 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

