Predictable dynamic program of timing of DNA replication in human cells
- PMID: 19767418
- PMCID: PMC2792175
- DOI: 10.1101/gr.094060.109
Predictable dynamic program of timing of DNA replication in human cells
Abstract
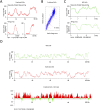
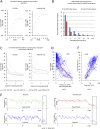
The organization of mammalian DNA replication is poorly understood. We have produced high-resolution dynamic maps of the timing of replication in human erythroid, mesenchymal, and embryonic stem (ES) cells using TimEX, a method that relies on gaussian convolution of massive, highly redundant determinations of DNA copy-number variations during S phase to produce replication timing profiles. We first obtained timing maps of 3% of the genome using high-density oligonucleotide tiling arrays and then extended the TimEX method genome-wide using massively parallel sequencing. We show that in untransformed human cells, timing of replication is highly regulated and highly synchronous, and that many genomic segments are replicated in temporal transition regions devoid of initiation, where replication forks progress unidirectionally from origins that can be hundreds of kilobases away. Absence of initiation in one transition region is shown at the molecular level by single molecule analysis of replicated DNA (SMARD). Comparison of ES and erythroid cells replication patterns revealed that these cells replicate about 20% of their genome in different quarters of S phase. Importantly, we detected a strong inverse relationship between timing of replication and distance to the closest expressed gene. This relationship can be used to predict tissue-specific timing of replication profiles from expression data and genomic annotations. We also provide evidence that early origins of replication are preferentially located near highly expressed genes, that mid-firing origins are located near moderately expressed genes, and that late-firing origins are located far from genes.
Figures




References
-
- Aladjem MI. Replication in context: Dynamic regulation of DNA replication patterns in metazoans. Nat Rev Genet. 2007;8:588–600. - PubMed
-
- Dalgaard JZ, Klar AJ. Orientation of DNA replication establishes mating-type switching pattern in S. pombe. Nature. 1999;400:181–184. - PubMed
-
- DePamphilis ML. The search for origins of DNA replication. Methods. 1997;13:211–219. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
