The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis
- PMID: 19767435
- PMCID: PMC2772484
- DOI: 10.1128/JB.00737-09
The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis
Abstract
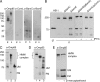
The evolutionarily conserved protein Omp85 is required for outer membrane protein (OMP) assembly in gram-negative bacteria and in mitochondria. Its Escherichia coli homolog, designated BamA, functions with four accessory lipoproteins, BamB, BamC, BamD, and BamE, together forming the beta-barrel assembly machinery (Bam). Here, we addressed the composition of this machinery and the function of its components in Neisseria meningitidis, a model organism for outer membrane biogenesis studies. Analysis of genome sequences revealed homologs of BamC, BamD (previously described as ComL), and BamE and a second BamE homolog, Mlp. No homolog of BamB was found. As in E. coli, ComL/BamD appeared essential for viability and for OMP assembly, and it could not be replaced by its E. coli homolog. BamE was not essential but was found to contribute to the efficiency of OMP assembly and to the maintenance of OM integrity. A bamC mutant showed only marginal OMP assembly defects, but the impossibility of creating a bamC bamE double mutant further indicated the function of BamC in OMP assembly. An mlp mutant was unaffected in OMP assembly. The results of copurification assays demonstrated the association of BamC, ComL, and BamE with Omp85. Semi-native gel electrophoresis identified the RmpM protein as an additional component of the Omp85 complex, which was confirmed in copurification assays. RmpM was not required for OMP folding but stabilized OMP complexes. Thus, the Bam complex in N. meningitidis consists of Omp85/BamA plus RmpM, BamC, ComL/BamD, and BamE, of which ComL/BamD and BamE appear to be the most important accessory components for OMP assembly.
Figures
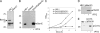

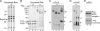


References
-
- Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99.
-
- Bos, M. P., V. Robert, and J. Tommassen. 2007. Biogenesis of the Gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191-214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

