Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis
- PMID: 19767450
- PMCID: PMC2937229
- DOI: 10.1165/rcmb.2009-0031OC
Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis
Abstract
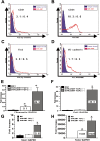
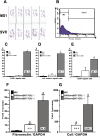
The pathological hallmark lesions in idiopathic pulmonary fibrosis are the fibroblastic foci, in which fibroblasts are thought to be involved in the tissue remodeling, matrix deposition, and cross-talk with alveolar epithelium. Recent evidence indicates that some fibroblasts in fibrosis may be derived from bone marrow progenitors as well as from epithelial cells through epithelial-mesenchymal transition. To evaluate whether endothelial cells could represent an additional source for fibroblasts, bleomycin-induced lung fibrosis was established in Tie2-Cre/CAG-CAT-LacZ double-transgenic mice, in which LacZ was stably expressed in pan-endothelial cells. Combined X-gal staining and immunocytochemical staining for type I collagen and alpha-smooth muscle actin revealed the presence of X-gal-positive cells in lung fibroblast cultures from bleomycin-treated mice. To explore the underlying mechanisms, by which loss of endothelial-specific markers and gain of mesenchymal phenotypes could be involved in microvascular endothelial cells, the effects of activated Ras and TGF-beta on the microvascular endothelial cell line MS1 were analyzed. Combined treatment with activated Ras and TGF-beta caused a significant loss of endothelial-specific markers, while inducing de novo mesenchymal phenotypes. The altered expression of these markers in MS1 cells with activated Ras persisted after withdrawal of TGF-beta in vitro and in vivo. These findings are the first to show that lung capillary endothelial cells could give rise to significant numbers of fibroblasts through an endothelial-mesenchymal transition in bleomycin-induced lung fibrosis model.
Figures

Comment in
-
Idiopathic pulmonary fibrosis is associated with endothelial to mesenchymal transition.Am J Respir Cell Mol Biol. 2010 Aug;43(2):129-30. doi: 10.1165/rcmb.2010-0044ED. Am J Respir Cell Mol Biol. 2010. PMID: 20651063 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

