Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress
- PMID: 19767530
- PMCID: PMC2781379
- DOI: 10.1152/ajpheart.00579.2009
Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress
Abstract
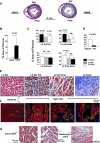
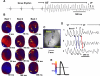
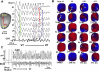
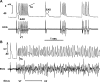
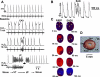
Oxidative stress with hydrogen peroxide (H(2)O(2)) readily promotes early afterdepolarizations (EADs) and triggered activity (TA) in isolated rat and rabbit ventricular myocytes. Here we examined the effects of H(2)O(2) on arrhythmias in intact Langendorff rat and rabbit hearts using dual-membrane voltage and intracellular calcium optical mapping and glass microelectrode recordings. Young adult rat (3-5 mo, N = 25) and rabbit (3-5 mo, N = 6) hearts exhibited no arrhythmias when perfused with H(2)O(2) (0.1-2 mM) for up to 3 h. However, in 33 out of 35 (94%) aged (24-26 mo) rat hearts, 0.1 mM H(2)O(2) caused EAD-mediated TA, leading to ventricular tachycardia (VT) and fibrillation (VF). Aged rabbits (life span, 8-12 yr) were not available, but 4 of 10 middle-aged rabbits (3-5 yr) developed EADs, TA, VT, and VF. These arrhythmias were suppressed by the reducing agent N-acetylcysteine (2 mM) and CaMKII inhibitor KN-93 (1 microM) but not by its inactive form (KN-92, 1 microM). There were no significant differences between action potential duration (APD) or APD restitution slope before or after H(2)O(2) in aged or young adult rat hearts. In histological sections, however, trichrome staining revealed that aged rat hearts exhibited extensive fibrosis, ranging from 10-90%; middle-aged rabbit hearts had less fibrosis (5-35%), whereas young adult rat and rabbit hearts had <4% fibrosis. In aged rat hearts, EADs and TA arose most frequently (70%) from the left ventricular base where fibrosis was intermediate ( approximately 30%). Computer simulations in two-dimensional tissue incorporating variable degrees of fibrosis showed that intermediate (but not mild or severe) fibrosis promoted EADs and TA. We conclude that in aged ventricles exposed to oxidative stress, fibrosis facilitates the ability of cellular EADs to emerge and generate TA, VT, and VF at the tissue level.
Figures




References
-
- Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 287: 996–1006, 1998 - PubMed
-
- Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004 - PubMed
-
- Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 112: 1266–1273, 2005 - PubMed
-
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

